DMS SupportingStatements_PartA Post DOTPRA Comments
DMS SupportingStatements_PartA Post DOTPRA Comments.docx
Examining Distraction and Driver Monitoring Systems to Improve Driver Safety
OMB: 2127-0770
Department of Transportation
Information Collection Request Supporting Statements: Part A
Examining Distraction and Driver Monitoring Systems to Improve Driver Safety
OMB Control No. 2127-New
Abstract:1
The National Highway Traffic Safety Administration (NHTSA) is seeking approval for this new information collection request (ICR) to allow NHTSA to conduct a study on driver monitoring systems (DMS). DMS refers to in-vehicle technology that can detect driver state and interact with the driver through the human-machine interface (the user interface that connects the driver to the vehicle). NHTSA is proposing to conduct a study to improve NHTSA’s understanding of the differences in approaches to driver state detection and the potential safety impacts of DMS. The voluntary study would involve recruiting licensed drivers for the following information collections as part of a simulated driving study: (1) an online eligibility questionnaire to determine eligibility for participation; (2) an appointment reminder confirmation process; (3) informed consent form for participation in Track A; (4) informed consent form for participation in Track B; (5) breathalyzer measurements to ensure continued eligibility for the study drives; (6) facial shape and height measurements; (7) sleep and food intake questionnaire; (8) Karolinska Sleepiness Scale measurements; (9) Track A study drives; (10) Track B study drives; and (11) Track B end of visit release agreement . The objective of this simulator driving study is to assess the ability of DMS to assess driver states (e.g., distracted, drowsy) and understand how differences in DMS impact the ability of a system to reliably assess different driving states. This study will add to the state of knowledge by experimentally collecting data to support a full assessment of the factors associated with DMS and the modeling of driver state based on sensor data.
Response to the collections of information is voluntary and the information collections are part of a one-time study, although some information collections are collected multiple times per respondent. Respondents are generally healthy individuals aged 18 and older. Efforts will be made to enroll a diverse age sample that broadly represents the age of the driving population and includes those at greater risk of crashing (e.g., less than 25 years of age and greater than 65 years of age). Additional efforts will be made to enroll individuals with diverse skin tones, oversampling those who rate themselves higher on the Fitzpatrick Skin Type Scale. The study will be reviewed and approved by the University of Iowa Institutional Review Board (IRB) before any data collection procedures begin.
Justification
Explain the circumstances that make the collection of information necessary. Identify any legal and administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
Circumstances making the data collection necessary
Driver engagement is a key factor in motor vehicle safety that allows the driver to observe and respond to various hazards. When drivers disengage and become inattentive to the driving environment, they are unable to observe hazards, which increases the risks associated with collisions. In 2013, NHTSA published the final version of the Visual-Manual NHTSA Driver Distraction Guidelines for In-Vehicle Electronic Devices. In the decade since, vehicle technologies and interfaces have evolved substantially and new research is needed to update the current state of knowledge on driver distraction, attention management, and distraction/risk assessment.
Specifically, NHTSA seeks to conduct new research on DMS. DMS use different data sources, including driver-facing cameras, vehicle inputs (e.g., steering wheel torque), driving performance (e.g., lane departures), and other measures (e.g., time on task) to monitor the state of drivers and are currently deployed in many production vehicles. Future production systems are also likely to use physiological sensors (e.g., heart rate) as tools to identify driver state more accurately.
NHTSA believes that DMS has the potential to improve motor vehicle safety by, for example, detecting and alerting drivers to distraction, drowsiness, or impairment, and then adjusting the vehicle technology to meet the needs of the driver or providing support in particular situations. Accordingly, it is important for NHTSA to be able to discern the differences in approaches to driver state detection, including the various sensor approaches. The planned study described in this ICR is designed to not only allow NHTSA to discern between different DMS but will also include the development of a test protocol for different DMS methodologies. The overall objective is to develop and deliver a methodology that will assess the ability of DMS to accurately determine driver state by collecting data to support a full assessment of the factors associated with DMS and modeling driver state based on sensor data in a driving simulator.
b. Statute authorizing the collection of information
NHTSA was established by the Highway Safety Act of 1970 (Pub. L. No. 91-605, §202(a), 84 Stat. 1713, 1739-40). Its mission is to reduce the number of deaths, injuries, and economic losses resulting from motor vehicle crashes on our nation’s highways. To further this mission, NHTSA conducts research as a foundation for the development of traffic safety programs. Specifically, NHTSA is authorized at 49 U.S.C. 30182 to: (1) conduct motor vehicle safety research, development, and testing programs and activities, including activities related to new and emerging technologies that impact or may impact motor vehicle safety and (2) collect and analyze all types of motor vehicle and highway safety data and related information to determine the relationship between motor vehicle or motor vehicle equipment performance and accidents involving motor vehicles and deaths or personal injuries resulting from those accidents. In carrying out these activities, NHTSA is authorized to undertake collaborative research and development projects with non-federal entities. This information collection supports the department’s strategic goal of safety.
Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
This request is for OMB approval of information collections related to a multipart study to assess the ability of DMS to assess driver states (e.g., distracted, drowsy) and how differences in DMS impact the ability of a system to reliably assess different driver states.
NHTSA has contracted with Westat, who has subcontracted with the University of Iowa Driving Safety Research Institute (DSRI), home of the National Advanced Driving Simulator (NADS), to conduct experimental research that empirically examines the ability of DMS to examine driver states to subsequently alert drivers to inattentive behavior and support safe re-engagement. Any information requested for collection is to aid in efforts to evaluate the ability of DMS to assess driver states. Information collection includes basic demographic information, questions to ensure adherence to study protocol, questions about general health and sleep/wake habits, and questions related to food, alcohol, nicotine, and caffeine consumption. This collection also includes information regarding how sleepy a person feels before and after they drive in the simulator, which will aid in the assessment of the DMS. General health questions are asked to screen out individuals to whom harm could occur while in the simulator, such as those with back or neck injuries. Questions about adherence to study protocol are to ensure minimized subsequent burden on individuals. For example, this could assist in mitigating scheduling an individual who could not abstain from alcohol prior to the visit and therefore must be sent home due to not meeting study eligibility criteria.
DSRI will conduct this new data collection effort as a subcontractor under a task order on an Indefinite Deliverable Indefinite Quantity contract between Westat and NHTSA. Study participation will be voluntary and solicited via email advertisements primarily through the DSRI Subject Registry and the University of Iowa Mass Mailing system. Registry members have been recruited through community events, email campaigns, participant referrals or word of mouth, and print, radio, and television advertisements. Individuals are also invited to the registry after completing a study at DSRI. Members of the registry primarily reside in Iowa and Illinois, though there are individuals from as far away as California. Being affiliated with a university also gives DSRI access to international students, staff, and faculty. Data will be collected by the research team at DSRI. Potential subjects will complete an online eligibility questionnaire (NHTSA Form 1718) to determine their eligibility to participate in the study and eligible subjects who are scheduled for an appointment will receive a reminder email with instructions and reminders for the visit (NHTSA Form 1799). The email also requests that subjects verify they have no current illness symptoms or feel unwell and that they have experienced no changes to their health. Eligible subjects 18+ will come to the DSRI where a research team member will review study procedures and obtain informed consent.
This study contains two tracks to assess DMS, and subjects may participate in Track A, Track B, or both. This allows for a balance between understanding how driver state detection changes within a diverse testing sample and within an individual across driver states. The overall sample will contain 80 data sets. Each track will have 40 completed data sets. Thus, the total sample size is anticipated to be 68 subjects and will include subjects that completed Track A only (n=28), Track B only (n=28), and those that completed both tracks (n=12). Track A will evaluate the ability of the DMS to assess distraction and Track B will evaluate the ability of the DMS to assess both drowsiness alone and distraction while drowsy.
Informed consent documents appropriate to the track (Track A or Track B) are approved by the University of Iowa Institutional Review Board (NHTSA Form 1730 and NHTSA Form 1731). If a subject participates in both tracks, they will sign both consent forms.
For Track A, subjects will arrive to DSRI at their scheduled visit time and complete the consent process and engage in pre-drive procedures (breathalyzer measurement, digital image of face, facial shape measurement, standing and seated height measurements, simulator training), then complete a familiarization drive of 20 minutes followed by a study drive of 60 minutes to assess the ability of the DMS to assess driver distraction. Subjects will complete pre- and post-drive sleepiness ratings using the Karolinska Sleepiness Scale (NHTSA Form 1719).
For Track B, subjects will arrive to DSRI at their scheduled visit time of 5:15 p.m. or 6:45 p.m., complete the consent process, complete a sleep and food intake questionnaire (NHTSA Form 1720) and breathalyzer measurement to confirm adherence to study protocol, have a digital image of the face taken so researchers can obtain RGB values to assess skin tone variability, have facial shape measurements taken, and have standing and seated height measurements taken. The subject will receive training on the simulator and study drives then complete a familiarization drive of 20 minutes followed by a baseline sleepiness rating (NHTSA Form 1719) and wellness check. Subjects will complete this sleepiness rating before and after every study drive and approximately every 30 minutes during their waiting period as well. After the familiarization drive, subjects will complete their first study drive of approximately 60 minutes (to assess alert state). Subjects will remain awake until their next study drive no earlier than 10:00 p.m. Subjects will complete two counterbalanced drives, one assessing drowsiness alone and one assessing drowsiness with distraction. These drives are each approximately 60 minutes and will occur back-to-back with a short restroom break between. Drives do not occur until the subject has been awake for at least 14 hours and shows signs of drowsiness. Subjects will not be permitted to drive, walk, or bike home and must have arranged for transportation home at the end of their overnight visit. Subjects will sign an agreement at the end of their visit that states the method of transportation home (NHTSA Form 1721). Subjects are provided compensation to aid with this transportation expense.
The information collected is to be used by DSRI researchers on behalf of NHTSA’s mission. This information will support the evaluation of the ability of DMS to assess driver states (e.g., distracted, drowsy) and reduce the incidence of serious and fatal injuries experienced by motorists.
Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also, describe any consideration of using information technology to reduce burden.
The DSRI subject registry, an electronic database of individuals who have participated in previous studies or expressed interest in participating, will be the primary method used to recruit subjects. Subjects will be recruited by email but can also call a research team member if they prefer. Questionnaire data will be collected electronically via REDCap, a secure web platform for building and managing online databases and surveys (https://redcap.icts.uiowa.edu/redcap/). The online eligibility questionnaire is done online via REDCap link emailed to potential subjects and can be done at the respondent’s convenience; the respondent can leave and come back to the questionnaire if desired should something interrupt them. Additionally, branching and display logic used in the questionnaire reduce the need for respondents to skip questions on their own if they don’t apply. The online eligibility questionnaire was adopted to allow for flexibility for respondents and has been very well received in previous studies conducted at DSRI.
Video and engineering data from the simulator are recorded and backed up automatically. Computer programs (MatLab and R) will be used to reduce simulator data to summary measures (e.g., means, standard deviations) and to perform statistical analyses and generate results figures.
Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purposes described in Item 2 above.
Existing research in this area does not consider more recent advances in technology nor do they consider existing types of systems that are designed to broadly detect impairment that is not linked a single type of impairment. This effort will help to address a research gap.
If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
The collection of information does not involve small businesses. Respondents are individuals who meet certain criteria and who volunteer for the study.
Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
Driver distraction is a substantial contributing factor to traffic crashes, injuries, and fatalities. Data from the Fatality Analysis Reporting System (FARS) shows that driver distraction was reported as a factor in the deaths of 3,142 people in 2020, contributing to 8 percent of all motor vehicle–related deaths2. Distraction, particularly visual distraction, has a clear negative impact on safety. Drowsiness also has negative safety consequences for driving. Although the precise number of drowsy driving crashes is difficult to estimate, recent analyses suggest drowsiness may contribute to up to 6 percent of all crashes and 21 percent of fatal crashes3. While DMS have the potential to reduce the contributions of distraction and drowsiness to vehicle fatalities, it is important to understand how different DMS approaches impact the accuracy and reliability of driver state detection, which has an impact on the ability of a system to provide timely and effectively countermeasures.
Evaluating DMS requires testing these systems in a manner that approximates the conditions in which they will be used, but in a controlled environment. That is, if these data are not collected, it will not be possible to assess the ability of DMS to assess driver states (e.g., distracted, drowsy) and how differences in DMS impact the ability of a system to reliably assess different driver states.
Explain any special circumstances that would cause an information collection to be conducted in a manner:
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records, for more than three years;
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secrets, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
There are no special circumstances that would cause this collection to be collected in a manner inconsistent with 5 CFR 1320.5(d)(2). Although this ICR involves respondents responding to the same information collection more often than quarterly, those responses are provided completely voluntarily and are necessary for this one-time study.
If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, required by 5 CFR 1320.8(d), soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken by the agency in response to the comments. Specifically address comments received on cost and hour burden. Describe efforts to consult with persons outside the agency to obtain their views.
NHTSA published a notice in the Federal Register with a 60-day public comment period to announce this proposed information collection on July 14, 2023 (88 FR 45269).
During the public comment period for the 60-day notice, NHTSA received four comments and one email. The first comment requested collection of data regarding circadian effects as related to school start times. This would involve subjects under the age of 18 and are not related to driver monitoring systems and is out of scope of the planned research project. The second comment expressed a dislike for driver monitoring systems as they expressed the opinion that DMS are a disciplinary tool rather than a safety tool. NHTSA respectfully disagrees with this opinion and believes DMS may be able to improve motor vehicle safety.
One email from the Alliance for Automotive Innovation asked if the research was in response to Sec. 24209 of the Infrastructure Investment and Jobs Act, 2021 (H.R. 3684; Pub. L. 117-58, enacted on November 15, 2021 and commonly referred to as the Bipartisan Infrastructure Law or BIL). NHTSA responded by email to the Alliance for Automotive Innovation and noted that this project does include elements that were funded by the IIJA/BIL legislation. The email response also encouraged submission of comments to regulations.gov and noted that NHTSA would provide responses to comments in a 30-day notice published in the Federal Register.
Two of the comments received were relevant to the burden and design of the study. The following summarizes the points brought up in those comments and NHTSA’s response.
The American Academy of Sleep Medicine (AASM) commended NHTSA for planning the current information collection. They found the assessment of both drowsiness and distraction while drowsy to be a progressive and necessary step in determining the utility of DMS as a tool for road safety.
The AASM commented that self-reported sleepiness may not always reflect an individual’s true level of sleepiness and recommended the inclusion of other objective measures of alertness, such as electroencephalography (EEG) or the psychomotor vigilance task (PVT) to strengthen the accuracy of collected drowsiness data. Response: The research team has used both EEG4 and PVT5 as part of prior drowsy driving research. We included the review of this data as part of preliminary steps in this research study. Specifically, we found a strong relationship between the Observer Rating of Drowsiness (ORD) and the Karolinska Sleepiness Scale (KSS) (r = 0.682, p < 0.001) and weak relationships between ORD and Psychomotor Vigilance Task (PVT) prior to the drive (r = 0.150, p < 0.001) and after the drive (r = 0.244, p < 0.001). Based on our prior published research, the inherent value of adding EEG is limited, but there are substantial increases to the burden (e.g., application/cleanup & driver distraction) that do not outweigh this benefit. Depending on the EEG system, applying the EEG to the participant’s scalp can range from 45 minutes to 120 minutes. The EEG may also interfere with the driver and cause additional distraction, discomfort, or prevent them from becoming immersed in the driving scenario, further reducing ecological validity. Recently, other researchers have investigated the associations between KSS, ORD, vehicle-based measures, and metrics from electrooculogram (EOG) and EEG6. KSS was associated with ORD, standard deviation of lateral position (SDLP), percentage of eyelid closure over the pupil over time (PERCLOS), EEG alpha power, EEG theta power, and percentage of time with slow eye movement. Interestingly, measures from the physiological sensors (i.e., EEG and EOG) displayed only weak and moderate associations. Given these considerations, we maintain that the KSS will produce sufficiently accurate data to support the goals of the data collection while minimizing participant burden. The KSS will be used to determine when drivers have achieved a certain level of drowsiness and thus, they will begin the drowsy drive. We anticipated participants will complete the KSS nine times prior to the drive. Drowsiness will be defined based on a combination of the participant being awake for a minimum of 14 hours and the KSS. The KSS will not be administered during the drive as this may influence driver’s levels of drowsiness. Drowsiness during the drive will be captured by measures derived from eye closures over the course of the drive (e.g., PERCLOS). Given that each approach to measuring drowsiness comes with inherent flaws, we are using a combination of measures to infer drowsiness based on a sleepiness scale to bookend drowsiness during the drive and use of eye measures (i.e., PERCLOS) to elucidate changes in drowsiness levels during the drive.
The AASM recommended that the information collection include an assessment of possible sleep disorders during the online eligibility questionnaire and advised excluding individuals with untreated sleep disorders from the study. Additionally, AASM recommended that the data collection include a measure of participant sleep quality in order to quantify contributing factors to drowsiness and driving performance; they suggested use of a participant sleep log and/or a three-day reporting of bedtimes, waketimes, estimate of the amount of time to fall asleep, number of awakenings, estimate of the amount of time awake during the awakenings, and daytime sleeping times and duration. Response: The proposed study procedures will capture wake and sleep time for the day preceding the study visit. We are not aware of any validated sleep log, and as additional measures would increase burden to participants, we have proposed to only ask targeted items that are known to influence drowsiness (i.e., wake time and sleep time) and can be used to provide measures for the analysis (i.e., hours of sleep and continuous time awake). The items that we ask participants are extracted from sleep logs and are variables that we could include in our statistical models. Since the sleep logs are not validated, we selected specific items, rather than using the entire log, as this reduces participant burden. Given that the focus of this research is on the manifestation of drowsiness (i.e., for the purpose of determining validity of DMS assessment) while driving in the general driving population, we did not propose collecting subjective evaluation of sleep quality in subjects which might be better addressed by NIH funded research, nor do we plan to exclude participation based on sleep disorders given that an estimated 9 to 15% of individuals have ongoing sleep disorders. A DMS will need to detect distraction and drowsiness, regardless of individual health conditions, and exclusion of these drivers could hinder the external validity of findings from this research. The presence of daytime drowsiness regardless of source will be collected using self-reported sleepiness via the KSS.
The AASM also requested clarification on how the data obtained from the study would be protected, particularly as it related to prevention of consequences for participants who are distracted while driving. The AASM also asked whether a certificate of confidentiality would be provided. Response: The study has received approval from the University of Iowa Institutional Review Board, which requires us to protect the participants’ anonymity. Respondents’ performance in the driving simulator will be deidentified and separated from any personally identifiable information. Certificates of confidentiality are generally not sought unless we are collecting data that would put the participants at legal risk, which is not the case in this study.
The National Association of Mutual Insurance Companies (NAMIC) commented that the use of the Fitzpatrick Skin Type Scale in the online eligibility questionnaire, which requires participants to self-rate, negates the uniformity of the scale. Further, NAMIC questions why the study intends to oversample participants who are rated higher on the scale (e.g., darker skin types). Response: The proposed self-rating of an applicant on the Fitzpatrick Skin Type Scale will be used to inform our study stratification and data collection logistics. The scale will be used to objectively quantify their skin pigmentation upon consenting and enrolling our study by a single rater. Additionally, the RGB values for skin tone will be captured during the visit via visual processing to provide an objective metric with greater gradation.
NAMIC also requested additional clarification on which driver monitoring system(s) will be used in the study. Response: The team will implement a sensor suite to provide the same types of signals available to a variety of types of DMS including vehicle and driver data. DSRI has existing relationships with technology suppliers that will be leveraged to provide necessary data. We do not propose to evaluate the algorithms from any technology suppliers, but instead focus on the utility of the underlying signals in detection.
Both AASM and NAMIC commented on the importance of recruiting participants from a large audience to ensure a sample that is representative and generalizable to a larger driving population. NAMIC noted their concerns related to the limited location (noting a 30-mile radius around Iowa City, IA), number of participants, and participant selection process. Response: A power analysis was conducted to estimate the sample size needed for the study. We agree that generalizability is important and must be balanced with the experimental aims of the research. Given that the research method utilizes a one-of-a-kind driving simulator, recruitment must be focused in the geographic area where it is housed. The plan is to maximize diversity of the sample within the limits of the proposed sample size through robust recruitment utilizing the existing registry which includes thousands of potential participants that includes the Cedar Rapids-Iowa City, IA CSA; Davenport-Moline, IA-IL CSA; Waterloo-Cedar Falls, IA MSA; Dubuque, IA MSA; Ottumwa, IA USA; Fort Madison-Keokuk, IA-IL-MO USA; Burlington, IA-IL USA; and Marshalltown, IA USA in addition to the surrounding rural areas. To expand the diversity of the overall sample, areas outside of Iowa City are being included in the recruitment approach. Additionally, participants who are not in the registry are not excluded from participating. No participants are excluded due to location so long as they are able to arrange safe transportation to/from the facility for the overnight visit. Prior research has shown that this can be done effectively, particularly when the study includes within-subject comparisons, which is one reason why we are including a subset of the sample in both tracks. As Iowa is less ethnically diverse than the US population overall, targeted recruitment will be performed to promote a more balanced sample based on the Fitzpatrick Skin Type Scale, which is also a crucial variable to include when assessing the capabilities of DMSs. The proposed self-rating of an applicant on the Fitzpatrick Skin Type Scale will be used to inform our study stratification and data collection logistics.
Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
Subjects will be offered $60 as compensation for completion of Track A (approximately 2 hours) and $270 as compensation for completion of Track B (approximately 9 hours). Pay will be pro-rated at $30/hour. Our past experience indicates that anything less than $25/hour total compensation would likely result in a failure to recruit enough subjects. With an overnight data collection and a desire to keep rate of pay consistent between studies, we chose $30/hour. In addition to the $270 compensation for completing the study visit, subjects in Track B will receive $70 to aid with transportation expenses due to the requirement to not drive, walk, or bike home and the burden of needing to arrange transportation. Previous studies conducted at DSRI have shown that $70 compensation for transportation expenses was sufficient to limit subject attrition and reimburse for third-party transportation costs.
Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy. If the collection requires a systems of records notice (SORN) or privacy impact assessment (PIA), those should be cited and described here.
All data will be treated with sensitivity and security considerations commensurate with its level of confidential content. The University of Iowa IRB will review all instruments, informed consent materials, and procedures to ensure that the rights of individuals participating in the study are safeguarded before recruitment for the study can begin. The IRB is a specially constituted review body established to protect the welfare of human subjects recruited to participate in research.
As stated in the informed consent forms, no individual results or personal information will be published. Published documents will provide only summary statistics that cannot be used to identify an individual. Links between individual names and study numbers will be securely stored according to the provisions of the University of Iowa Institutional Review Board.
Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
We inquire about general health history as a safety precaution to screen for conditions that could prove dangerous to the individual or a research staff member. The responses to these health history questions are not recorded or reported on in the final dataset but are used as a screening tool.
Provide estimates of the hour burden of the collection of information on the respondents and estimates of the annualized labor cost to respondents associated with that hour burden.
The annual estimated time burden to complete the collection of information is 175 hours and an annual opportunity cost of $5,159 over the three-year approval period.
To minimize the burden for those individuals who complete both tracks and to ensure the research team can find individuals eligible for both tracks, the eligibility questionnaire is administered only once and includes eligibility questions for both Track A and Track B. An estimated 600 individuals will initiate a response to the online eligibility questionnaire. This is based on recent response rates to studies conducted at DSRI using similar age ranges and study criteria, then rounded to the nearest 100. These can be seen in Table 1 below (Study 5 was the only recent overnight data collection for which data was available, but the others are included to show the range seen in daytime data collections). An estimated 300 individuals will complete the online eligibility questionnaire and be determined initially eligible for the study. The other 300 are believed to either self-terminate the online eligibility questionnaire prior to completion or do not meet criteria and are directed to a message stating such.
Table 1: Recent Study Response Rates for Justification Purposes
-
Study 1
Study 2
Study 3
Study 4
Study 5
Total Responses to Pre-Screening
575
515
638
496
592
Total Initially Eligible based on Response
(% of Total Responses)258 (44.8%)
256 (49.7%)
353 (55.3%)
311 (62.7%)
260 (43.9%)
Total Enrolled into Study (% of Total Responses)
144 (25%)
132 (25.6%)
82 (12.9%)
132 (26.6%)
121 (20.4%)
Of the 300 individuals that complete the online eligibility questionnaire, an estimated 255 would be eligible to complete both Track A and Track B and an estimated 45 would be eligible for only Track A. This is due to not meeting requirements related to sleep patterns for Track B. To assess drowsiness, subjects must not experience peak wakefulness in the night hours. Those individuals who identify as “night owls”, or roughly 10-15% of the population7, will be excluded from Track B. Further attrition is expected throughout the information collection. To see a visualization of the respondent pools and how they are affected by attrition at the various stages of the study, see Figure 1.
Figure 1: Anticipated Respondents throughout the Information Collection
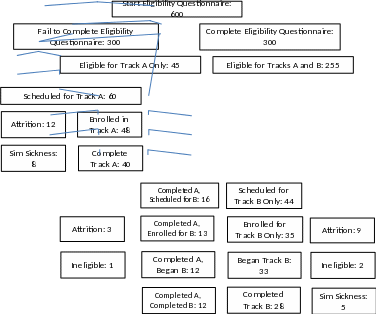
Track A will run prior to Track B. The pool of eligible respondents will be contacted to schedule an appointment for Track A. It is estimated that 60 respondents will be scheduled for a Track A appointment. Assuming cancelation rates similar to recent studies run post-COVID, we expect to lose roughly 20% of those scheduled prior to enrollment due to not showing up for appointments or last-minute cancelations. From here, it is expected that 48 respondents will sign a consent form, or enroll, in Track A. Further attrition is expected due to simulator sickness. This results in the final data set of 40 respondents for Track A. A subset of these individuals will also complete Track B.
Track B will be comprised of two subsets of respondents. The first subset is those respondents who have not yet been enrolled, and the second is a subset of individuals who also completed Track A. After recruitment ends for Track A, the pool of eligible respondents for Track B will be contacted to schedule an appointment for Track B only. It is estimated that 44 respondents will be scheduled for a Track B appointment. Assuming cancelation rates similar to recent studies run post-COVID, we expect to lose roughly 20% of those scheduled prior to enrollment due to no show appointments, last-minute cancelations, or issues with transportation. From here, it is expected that 35 respondents will sign a consent form, or enroll, in Track B. Further attrition is expected due to factors such as simulator sickness or failure to meet eligibility criteria (e.g., positive breathalyzer reading, consumption of caffeine, afternoon nap). This results in a data set of 28 respondents for Track B.
For the subset of individuals who completed Track A who will also complete Track B, it is estimated that 16 individuals will be scheduled for a Track B appointment upon completion of their Track A appointment. Assuming the same 20% cancelation rate, we expect 13 individuals will sign a consent form, or enroll, in Track B. Given these individuals successfully completed Track A, we expect no attrition due to simulator sickness. However, it is still possible that a respondent may fail to meet eligibility criteria. This results in 12 complete and brings the total number of Track B datasets to 40.
The answer to Question 2 in this supporting statement describes the components of the study and the order of those components. Below are estimates of the individual components of the study. For calculations of both time burden and opportunity cost, the above-described sets of respondents have been separated into individual respondent pools for Track A and Track B. For an outline of the approximate Track B visit timeline, see Appendix A.
NHTSA Form 1718, Online Eligibility Questionnaire ensures that respondents are eligible for participation. It is estimated that 600 individuals will initiate a response to the online eligibility questionnaire one time, with 300 individuals completing the questionnaire. Individuals that initiate a response to the online eligibility questionnaire but do not complete the questionnaire will average approximately 5 minutes per response. Individuals that complete the online eligibility questionnaire will spend 15 minutes to complete the questionnaire. This results in an average of 10 minutes for 600 respondents, with a single response each.
NHTSA Form 1799, Appointment Reminder Confirmation Process contains the reminder email and information provided to the subject related to their visit. The email is sent one time to the respondent 24 hours prior to the scheduled visit. The respondent does not have to respond to any specific questions but is asked to contact the research team if there has been a change in health or if they are currently experiencing any symptoms of illness or feeling unwell. To confirm the visit, the respondent will click on a link that takes them to an information page that contains reminders and instructions for the visit. The researchers expect to schedule 60 individuals for Track A: 44 will receive just one confirmation email and 16 will receive the confirmation email twice. Forty-four individuals will receive the confirmation email for Track B. Therefore, 104 respondents will receive the confirmation email an average of 1.15 times. The respondents will take approximately 5 minutes to read the information one time and respond if necessary.
NHTSA Form 1730, Track A Informed Consent describes the study procedures for Track A subjects and any potential risks of participation. The researchers expect 48 respondents will complete the Track A Informed Consent document prior to their participation in the Track A study drive. Respondents will take approximately 15 minutes to complete the form one time.
NHTSA Form 1731, Track B Informed Consent describes the study procedures for Track B subjects and any potential risks of participation. The researchers expect 48 respondents will complete the Track B Informed Consent document prior to their participation in the Track B study drives (35 respondents for Track B only and 13 who also completed Track A). Respondents will take approximately 15 minutes to complete the form one time.
Breathalyzer Measurement allows the researchers to test for the presence of alcohol and serves as an eligibility confirmation tool. Eighty-three respondents will need to take the breathalyzer measurement. Thirteen of the respondents will take the breathalyzer measurement twice (those respondents participating in both Track A and Track B). The remaining 35 respondents of Track A only and the 35 respondents for Track B only will take the breathalyzer measurement once. A total of 96 measurements will be collected over the 83 respondents, thus, the frequency of response is 1.16. Respondents will take approximately 3 minutes to complete the task.
Facial Shape and Height Measurement allows the researchers to obtain ratios of facial dimensions and height to use as a covariate in analysis of the DMS data. During this time, a researcher will also take a digital image of the face to collect RGB values for assessment of skin tone. This measurement is taken after eligibility confirmation. As such, 48 of the Track A respondents will have the measurement taken prior to participation in Track A. Of those respondents that completed Track A and are enrolled in Track B (13 people), it is expected that 1 respondent will not be eligible to continue, thus 12 Track A and B respondents will have the facial measurements taken again prior to Track B. Researchers also expect to lose 2 respondents for lack of eligibility from the 35 enrolled Track B only respondents, thus only 33 would have the facial measurement conducted prior to Track B. This results in 36 respondents with the face measurement for Track A only, 12 respondents with two face measurements, and 33 respondents with one measurement for Track B and a total of 93 facial shape and height measurements. These 81 respondents will have these measurements taken an average of 1.15 times. The reason for two measurements from the 12 respondents prior to both Track A and Track B participation is that it is possible to see variance in the RGB values due to the presence or absence of alterations such as makeup between visits. Respondents will take approximately 7 minutes to complete the task.
NHTSA Form 1719, Karolinska Sleepiness Scale evaluates self-rated sleepiness on a scale from 1 to 10, recorded before and after each study drive. It is expected that 81 respondents will rate themselves on this scale. The 8 Track A respondents will take this assessment once at the beginning but are expected not to complete the drive due to simulator sickness and therefore will not rate themselves a second time. Forty respondents will complete the Track A drive and therefore will rate their sleepiness twice. Of those 40, 12 will also complete Track B and thus rate themselves another 14 times. The 33 Track B only respondents will take this assessment up to 14 times. However, it is expected that 5 respondents will be unable to complete the entirety of the visit due to simulator sickness and will therefore have fewer administrations of the scale. For these 5 respondents, we will assume 7 responses to accommodate attrition at different points in the visit. The 28 that will complete Track B will rate their sleepiness 14 times. Overall, this results in 81 people rating their sleepiness and a total of 683 responses. Thus 81 people will take this an average of 8.43 times. Each rating will take one minute.
Track A Study Drive will consist of the Training Presentation, the Familiarization Drive and the Study Drive. The Training Presentation provides a summary of the simulator, the familiarization drive, the study drive(s), and study tasks prior to the subject entering the simulator. This is expected to take 10 minutes. The Familiarization Drive acclimates the subject to the driving simulator and screens for simulator sickness. This is expected to take 20 minutes to complete the task. The Study Drive is the main experiment in the driving simulator to assess the ability of the DMS to assess driver state. The Track A study drive takes approximately 60 minutes. There is no information collection directly from the respondent in this Track A Study Drive, but burden is calculated for time involved in the study. The three components will take 90 minutes. There are 48 respondents who will participate in this and will participate once. However, it is expected that 8 respondents will experience simulator sickness and be unable to complete these procedures. Assuming roughly 75% of these 8 respondents cannot proceed to the study drive due to simulator sickness and 25% begin but do not complete the study drive, this results in 6 respondents at 30 minutes, 2 respondents at 60 minutes, and 40 respondents at 90 minutes. This results in an average time of 81.25 minutes.
NHTSA Form 1720, Sleep and Food Intake records the time and nature of last meal, caffeine, nicotine, alcohol, and duration and times of sleep and naps. This form is specific to Track B respondents and they will each provide this information once. Forty-eight respondents will provide information (13 respondents participating in both Track A and B and 35 participating in Track B only). This intake will take approximately 5 minutes to complete.
Track B Study Drive will consist of the Training Presentation, the Familiarization Drive, three Study Drives, and the Wait Time. The Training Presentation provides a summary of the simulator, the familiarization drive, the study drive(s), and study tasks prior to the subject entering the simulator. This is expected to take 10 minutes. The Familiarization Drive acclimates the subject to the driving simulator and screens for simulator sickness. This is expected to take 20 minutes to complete the task. The three Study Drives are the main experiment in the driving simulator to assess the ability of the DMS to assess driver state. The Track B study drives takes approximately 60 minutes each. There is also a Wait Time for Track B respondents. This is the time between subjects completing the first study drive and beginning the second and third study drives (done back-to-back), during which the respondent can engage in tasks such as reading or watching movies but cannot sleep. This wait time will differ depending on whether the subject is scheduled first or second for the visit and is to ensure that subjects are drowsy for the second and third study drives. The average wait time for respondents will be 225 minutes; however, the respondents will need to utilize the Karolinska Sleepiness scale an average of eight times during this wait. To avoid double counting, eight minutes has been deducted from the average wait time, bringing the average to 217 minutes. There is no information collection directly from the respondent in this Track B Study Drive (aside from the sleepiness scale which is counted previously), but burden is calculated for time involved in the study. The entirety of Track B Study Drive is 427 minutes. There are 45 respondents who will participate in this and will participate once (12 respondents participating in both Track A and B and 33 participating in Track B only). However, it is expected that 5 respondents will experience simulator sickness and be unable to complete these procedures. Assuming roughly 75% of these 5 respondents cannot proceed to the first study drive due to simulator sickness and 25% begin but do not complete the second study drive, this results in 4 respondents with a burden of 30 minutes, 1 respondent with a burden of 277 minutes, and 40 respondents with a burden of 427 minutes. This results in an average time of 388.38 minutes.
NHTSA Form 1721, End of Visit Release Agreement ensures that subjects in Track B will not drive, bike, or walk home after the visit, and that they agree not to drive until well-rested. If a respondent attends the appointment, they will sign this form. Therefore, 48 respondents will complete this form (all respondents enrolled will need to complete the form regardless of completion) and it will take approximately 2 minutes to complete.
Cost calculation is opportunity cost as this is time for an individual rather than costs to a company paying labor costs. To calculate the opportunity cost associated with the forms and other relevant activities necessary for this collection of new information, NHTSA looked at average hourly earnings for employees on private nonfarm payrolls. NHTSA estimated the total opportunity costs associated with these burden hours by looking at the average wage for total private employees on private nonfarm payrolls. The Bureau of Labor Statistics (BLS) estimates that the average hourly wage for this group is $32.36.8
Table 2 provides detailed information for the total burden hours and opportunity costs for the study based on the full methodology and test matrix.
Table 2: Total Burden Estimates
Study Component |
Number of Respond-ents |
Frequency of Response |
Total Responses |
Time per Response |
Cost Per Response $32.36/ Hour |
Total Estimated Burden (Rounded) |
Total Opportunity Costs (rounded) |
Online Eligibility Questionnaire (Form 1718) |
600 |
1 |
600 |
10 min |
$5.39 |
100 hrs |
$3234.00 |
Appointment Reminder Confirmation Process (Form 1799) |
104 |
1.15 |
120 |
5 |
$2.70 |
10 hrs |
$324.00 |
Breathalyzer Measurement |
83 |
1.16 |
96 |
3 |
$1.62 |
4.8 hrs |
$155.52 |
Facial Shape and Height Measurement |
81 |
1.15 |
93 |
7 |
$3.78 |
10.85 hrs |
$351.54 |
Karolinska Sleepiness Scale (Form 1719) |
81 |
8.43 |
683 |
1 |
$0.54 |
11.38 hrs |
$368.82 |
Track A Informed Consent (Form 1730) |
48 |
1 |
48 |
15 |
$8.09 |
12 hrs |
$388.32 |
Track
A |
48 |
1 |
48 |
81.25 |
$43.82 |
65 hrs |
$2103.36 |
Track B Informed Consent (Form 1731) |
48 |
1 |
48 |
15 |
$8.09 |
12 hrs |
$388.32 |
Sleep & Food Intake (Form 1720) |
48 |
1 |
48 |
5 |
$2.70 |
4 hrs |
$129.44 |
Track
B |
45 |
1 |
45 |
388.38 |
$209.47 |
291.29 hrs |
$9425.98 |
End of Visit Release Agreement (Form 1721) |
48 |
1 |
48 |
2 |
$1.08 |
1.6 hrs |
$51.78 |
Total Burden |
|
|
|
|
|
522.92 hrs |
$16,921.08 |
Table 3 provides an annual breakdown of the burden hours and opportunity costs. There may be slight differences if the reader were to average Table 2 or triple Table 3 due to necessary rounding of figures; however, Table 3 corresponds to the Information Collection data in ROCIS for that necessary rounding. Note, Table 3 figures are the average over the three-year approval period.
Table 3. Annual Burden Estimates
Study Component |
Annual Number of Respond-ents |
Frequency of Response |
Annual Responses |
Time per Response |
Cost Per Response $32.36/Hour) |
Annual Estimated Burden (Rounded) |
Annual Opportunity Costs (rounded) |
Online Eligibility Questionnaire (Form 1718) |
200 |
1 |
200 |
10 min |
$5.39 |
33 hrs |
$1078 |
Appointment Reminder Confirmation Process (Form 1799) |
35 |
1.15 |
40 |
5 |
$2.70 |
3 hrs |
$108 |
Breathalyzer Measurement |
28 |
1.16 |
32 |
3 |
$1.62 |
2 hrs |
$52 |
Facial Shape and Height Measurement |
27 |
1.15 |
31 |
7 |
$3.78 |
4 hrs |
$117 |
Karolinska Sleepiness Scale (Form 1719) |
27 |
8.43 |
228 |
1 |
$0.54 |
4 hrs |
$123 |
Track A Informed Consent (Form 1730) |
16 |
1 |
16 |
15 |
$8.09 |
4 hrs |
$129 |
Track
A |
16 |
1 |
16 |
81.25 |
$43.82 |
22 hrs |
$221 |
Track B Informed Consent (Form 1731) |
16 |
1 |
16 |
15 |
$8.09 |
4 hrs |
$129 |
Sleep & Food Intake (Form 1720) |
16 |
1 |
16 |
5 |
$2.70 |
1 hr |
$43 |
Track
B |
15 |
1 |
45 |
388.38 |
$209.47 |
97 hrs |
$3142 |
End of Visit Release Agreement (Form 1721) |
16 |
1 |
16 |
2 |
$1.08 |
1hr |
$17 |
Total Burden |
|
|
626 |
|
|
175 hrs |
$5,159 |
Provide an estimate of the total annual cost burden to respondents or record keepers resulting from the collection of information. Do not include the cost of any hour burden already reflected in the response provided in question 12.
The respondents are not expected to incur any reporting or recordkeeping cost from the information collection. The only costs associated with any of the information collections is the cost for travel to and from DSRI, which is associated with each of the study drives. We estimate that 83 respondents will travel to DSRI for each of the two tracks, though 13 respondents will travel for both tracks resulting in 96 round trips. We expect most subjects to be traveling locally, within 30 miles from the test facility. Maximally, we estimate a round trip distance from subjects’ starting destination to DSRI to be 60 miles. The standard mileage rate for business-related driving in 2023 is 65.5 cents per mile driven, or $39.30 for 60 miles driven. Therefore, we estimate the maximum travel costs associated with Track A Study Drive to be $1,886 (48 respondents × $39.30 = $1,886.40). We estimate that the total transportation costs will be higher for subjects in Track B, who will not be permitted to walk, bike, or drive when leaving the test facility. Previous overnight studies conducted at DSRI have shown that $70 compensation for transportation expenses was sufficient to limit subject attrition and offset costs of third-party transportation. Accordingly, we estimate the travel costs associated with Track B Study Drive to be $3,360 (48 respondents × $70 = $3,360). The total costs for this ICR are estimated to be $5,246 ($1,886 + $3,360). These transportation costs are offset by subject compensation. For subjects in Track B, who will not be permitted to walk, bike, or drive when leaving the test facility, an additional $70 will be provided to offset the costs of finding alternative transportation.
Provide estimates of annualized costs to the Federal government. Provide a description of the method used to estimate cost, which should include quantification of hours, operational expenses (such as equipment, overhead, printing, and support staff), and any other expense that would not have been incurred without this collection of information.
Total estimated cost to the Government for this one-time information collection is $327,518. The annualized cost across a three-year approval period is $109,173.
Table 4. Estimated Cost to Federal Government
Direct Labor |
Hours |
Rates |
Total |
Associate Researcher |
160 |
$42.01 |
$6,722 |
Assistant Researcher |
240 |
$33.47 |
$8,033 |
Assistant Researcher |
1328 |
$28.41 |
$37,730 |
Simulator Operator, Systems & Operations |
160 |
$38.44 |
$6,150 |
Subtotal Direct Labor |
1888 |
|
$58,635 |
Fringe Benefits |
|
|
$12,910 |
Total Direct Labor |
|
|
$71,544 |
Computer Charges |
|
$6,457 |
|
Project Specific Supplies |
|
$586 |
|
Subject Payments |
|
$15,400 |
|
Simulator usage |
|
$182,307 |
|
Total Other Direct Costs |
|
|
$204,751 |
SUBTOTAL ALL DIRECT COSTS |
|
|
$276,295 |
Indirect Costs (at 54.5%) |
|
|
$51,223 |
TOTAL PRICE |
|
|
$327,518 |
Explain the reasons for any program changes or adjustments reported on the burden worksheet. If this is a new collection, the program change will be entire burden cost and number of burden hours reported in response to questions 12 and 13. If this is a renewal or reinstatement, the change is the difference between the new burden estimates and the burden estimates from the last OMB approval.
The collection of this information is associated with a new project. Therefore, there is no program change from an existing collection. As stated in Question 12, the annual burden time for this new collection is 175 hours and the annual opportunity cost for the collection is $5,159.
For collections of information whose results will be published, outline plans for tabulation and publication. Address any complex analytical techniques that will be used. Provide the time schedule for the entire project, including beginning and ending dates of the collection of information, completion of report, publication dates, and other actions as applicable.
Findings from the data collection effort will be compiled in a final report that will undergo agency review at NHTSA and will include an Executive Summary, Background, Introduction, Methodology, Results, and Conclusions sections.
The current plan is for a draft final report in 2025 with publication of a final report and final briefing in 2025 or early 2026. The final report will provide summary statistics and tables as well as the results of statistical analysis of the information, but it will not include any personal identifiable information.
If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
NHTSA will display the expiration date for OMB approval.
Explain each exception to the topics of the certification statement identified in "Certification for Paperwork Reduction Act Submissions." The required certifications can be found at 5 CFR 1320.9.9
No exceptions to the certification are made.
Appendix A: Approximate Visit Timeline for Track B
-
Task
Subject 1
Subject 2
Consent
17:15-17:35
18:45-19:00
Eligibility Procedures and Measurements
17:35-17:50
19:05-19:20
Training PPT
17:50-18:00
19:20-19:30
Familiarization Drive and KSS
18:00-18:20
19:30-19:50
Study Drive 1 and KSS
18:20-19:30
19:50-21:00
Waiting Period with KSS every 30 minutes
19:30-22:50
21:00-1:20
Study Drive 2 and Pre/Post-KSS
22:50-0:00
1:20-2:30
Restroom and Short Break
0:00-0:05
2:30-2:35
Study Drive 3 and Pre/Post-KSS
0:05-1:15
2:35-3:45
Visit End Procedures
1:15-1:20
3:45-3:50
1 The Abstract must include the following information: (1) whether responding to the collection is mandatory, voluntary, or required to obtain or retain a benefit; (2) a description of the entities who must respond; (3) whether the collection is reporting (indicate if a survey), recordkeeping, and/or disclosure; (4) the frequency of the collection (e.g., bi-annual, annual, monthly, weekly, as needed); (5) a description of the information that would be reported, maintained in records, or disclosed; (6) a description of who would receive the information (e.g., DOT, first responders, the general public, etc.); (7) the purpose of the collection; and (8) if a revision, a description of the revision and the change in burden.
2 Stewart, T. (2022, March). Overview of motor vehicle crashes in 2020 (Report No. DOT HS 813 266). NHTSA.
3 Tefft, B. C. (2014). Prevalence of motor vehicle crashes involving drowsy drivers, United States, 2009-2013. https://www.aaafoundation.org/sites/default/files/AAAFoundation-DrowsyDriving-Nov2014.pdf
4 Brown, T., Johnson, R., & Milavetz, G. (2013). Identifying Periods of Drowsy Driving Using EEG. Annals of Advances in Automotive Medicine, 57, 99. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3861841/; Brown, T., Lee, J., Schwarz, C., Fiorentino, D., McDonald, A., Traube, E., & Nadler, E. (2013). Detection of Driver Impairment from Drowsiness. 23rd International Technical Conference on the Enhanced Safety of Vehicles, Seoul, South Korea.; Brown, T., Lee, J., Schwarz, C., Fiorentino, D., & McDonald, A. (2014). Assessing the Feasibility of Vehicle-Based Sensors to Detect Drowsy Driving. (DOT HS 811 886). Washington, DC: National Highway Traffic Safety Administration Retrieved from http://www.nhtsa.gov/DOT/NHTSA/NVS/Crash%20Avoidance/Technical%20Publications/2014/811886-Assess_veh-based_sensors_4_drowsy-driving_detection.pdf
5 McDonald, A. D., Lee, J. D., Schwarz, C., & Brown, T. L. (2018). A Contextual and Temporal Algorithm for Driver Drowsiness Detection. Accident Analysis & Prevention.
6 Uchiyama, Y., Sawai, S., Omi, T., Yamauchi, K., Tamura, K., Sakata, T., Nakajima, K., & Sakai, H. (2023). Convergent validity of video-based observer rating of drowsiness, against subjective, behavioral, and physiological measures. PLoS one, 18(5), e0285557.
7 Räihä, T., Nerg, I., Jurvelin, H., Conlin, A., Korhonen, M., & Ala-Mursula, L. (2021). Evening chronotype is associated with poor work ability and disability pensions at midlife: a Northern Finland Birth Cohort 1966 Study. Occupational and Environmental Medicine, 78(8), 567-575. ; Breus, M. (2016). The Power of when: Discover Your Chronotype--and the Best Time to Eat Lunch, Ask for a Raise, Have Sex, Write a Novel, Take Your Meds, and More. Little, Brown Spark.; Pacheco, D., & Rehman, A. (2023, 16 November 2023). Chronotypes: Definition, Types & Effect on Sleep. Sleep Foundation. Retrieved 18 January from https://www.sleepfoundation.org/how-sleep-works/chronotypes#references-83113
8 See Table B-3 Average hourly and weekly earnings of all employees on private nonfarm payrolls by industry sector, seasonally adjusted, for August 2022, available at https://www.bls.gov/news.release/empsit.t19.htm (accessed November 23, 2022).
9 Specifically explain how the agency display the OMB control number and expiration date and will inform potential respondents of the information required under 5 CFR 1320.8(b)(3): the reasons the information is planned to be and/or has been collected; the way such information is planned to be and/or has been used to further the proper performance of the functions of the agency; an estimate, to the extent practicable, of the average burden of the collection (together with a request that the public direct to the agency any comments concerning the accuracy of this burden estimate and any suggestions for reducing this burden); whether responses to the collection of information are voluntary, required to obtain or retain a benefit (citing authority), or mandatory (citing authority);the nature and extent of confidentiality to be provided, if any (citing authority); and the fact that an agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Roach, Callie (NHTSA) |
| File Modified | 0000-00-00 |
| File Created | 2025-05-19 |
© 2025 OMB.report | Privacy Policy