Inclusion Enrollment Form
The Inclusion Enrollment Report Form
Attachment 2B_Inclusion Enrollment Report Instructions_Competing and noncompeting_FORMS-I
Inclusion Enrollment Form
OMB: 0925-0770
Competing Application Instructions
G.500 - PHS Human Subjects and Clinical Trials Information (OMB # 0925-0001, expiration date 01/31/2026)
2.9. Inclusion Enrollment Report(s) (OMB # 0925-0770, expiration date 09/30/2024)
Who must complete the Inclusion Enrollment Report(s):
An Inclusion Enrollment Report is required for all human subjects studies unless, on Question 1.3 “Exemption Number,” you selected only Exemption 4 and no other exemptions.
Using the Inclusion Enrollment Report:
Each proposed study, unless it falls under Exemption 4, must contain at least one Inclusion Enrollment Report (IER). However, more than one IER per study is allowed.
Once you have added an IER for a given study, you may edit, remove, or view it.
Note: You can add a maximum of 20 IERs per Study Record. These can be a combination of planned and cumulative reports.
Multi-site studies: Generally, if the application includes a study recruiting subjects at more than one site/location, investigators may create one IER or separate, multiple IERs to enable reporting by study or by site, depending on the scientific goals of the study and whether monitoring of inclusion enrollment would benefit from being combined or separated. At a minimum, participants enrolled at non-U.S. sites must be reported separately from participants enrolled at U.S. sites, even if they are part of the same study. Please review the NOFO to determine whether there are any other specific requirements about how to complete the IER.
Duplicative Inclusion Reports: It is important that the IER for a given study be associated with only one application and be provided only once in a given application (e.g., do not submit the same IER on both the data coordinating center and the research site). If submitting individual application(s) as part of a network or set of linked applications, please provide the IER with the individual site applications unless otherwise directed by the NOFO.
Renewal applications: When preparing a renewal (or resubmission of a renewal), investigators should provide a narrative description regarding the cumulative enrollment from the previous funding period(s) as part of the progress report section of the research strategy attachment in the application. The IER should NOT be used for this purpose. If a given study will continue with the same enrollment or additional enrollment, or if new studies are proposed, provide a new IER for each as described in the instructions below.
Resubmission applications: If IERs were provided in the initial submission application, and if those studies will be part of the resubmission application, complete the IER and submit again with the resubmission application, regardless of whether the enrollment has changed or not. Also, provide any new (additional) IERs.
Revision applications: Provide an IER if new studies are planned as part of the Revision and they meet the NIH definition for clinical research.
-
Additional Instructions for Multi-project:
For multi-project applications with studies that are self-contained within a single component:
Other Component: Include the IER(s) with the component(s) that involves the study(s), unless otherwise directed by the NOFO.
For multi-project applications with studies that span components:
Overall Component: Should the study span more than one component, include the IER with the Study Record in the Overall Component and insert a comment in the comment field of the IER to indicate what other components it is associated with.
For more information:
Refer to the Inclusion of Women and Minorities as Participants in Research Involving Human Subjects.
1. Inclusion Enrollment Report Title
The “Inclusion Enrollment Report Title” field is required.
The “Inclusion Enrollment Report title can have a maximum of 600 characters.
Enter a unique title for each IER. The title should indicate specific criteria that uniquely identify each report. If the Project Title is pre-populated, you may edit it so that each IER title is unique.
2. Using an Existing Dataset or Resource?
The “Using an Existing Dataset or Resource” question is required.
If the study involves analysis of an existing dataset or resource (e.g., biospecimens) only, answer “Yes” to this question. If the study involves prospective recruitment or new contact with participants answer “No” to this question. Use separate IERs for studies involving use of existing datasets or resources only and for studies that involve prospective recruitment or new contact with study participants.
For additional guidance on what is considered an existing dataset, refer to the NIH FAQs on Inclusion - Basis of Sex/Gender and Race/Ethnicity.
3. Enrollment Location Type (Domestic/Foreign)
The “Enrollment Location Type” field is required.
Select whether the participants described in the IER are based at a U.S. (Domestic) or at a non-U.S. (Foreign) site. Participants at U.S. and non-U.S. sites must be reported separately (i.e., on separate IERs), even if it is for the same study.
For additional guidance on how to complete the IER if you will be working with non-U.S. populations, refer to these FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
4. Enrollment Country(ies)
The “Enrollment Country(ies)” field is optional.
Indicate the country or countries in which participants will be enrolled. Multiple U.S. sites can be reported together in one IER. Foreign countries can be reported together in one IER. However, you must use separate IERs for U.S. and non-U.S. sites. You can add up to 200 countries per IER.
5. Enrollment Location(s)
The “Enrollment Location(s)” field is optional.
Indicate the type of enrollment location (e.g., hospital, university, or research center), not the name of the enrollment location.
Enrollment locations are typically where the research is conducted, and can be different from the recruitment site.
6. Comments
Your comments are limited to 500 characters.
Enter information you wish to provide about this IER. This includes, but is not limited to, addressing information about distinctive subpopulations if relevant to the scientific hypotheses being studied. If inclusion monitoring is conducted on another study or NIH grant (e.g., data coordinating center or research site), please indicate here.
Revision applications: If there are no updates to the IER(s) in your original grant application, do not include an IER in your Revision application. Instead, provide a comment in this field to the effect that previous IER(s) are still applicable. If you are revising the IER(s) in your original grant application, provide a comment here to that effect.
-
Additional Instructions for Multi-project:
For multi-project applications with studies that span components:
Overall Component: Should the study span more than one component, include the IER with the Study Record in the Overall Component and insert a comment here in the comment field to indicate what other components it is associated with.
Planned
Who must complete planned enrollment tables:
All studies must enter planned enrollment counts unless your proposed study will use only an existing dataset or resource. Planned enrollment generally means that individuals will be recruited into the study and/or that individuals have already been recruited and continue to be part of the study.
For more information about what is considered an existing dataset or resource for inclusion policy, see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both American Indian/Alaska Native and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both American Indian/Alaska Native and Hispanic or Latino.
Asian:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Asian and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Asian and Hispanic or Latino.
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and Hispanic or Latino.
Black or African American:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Black or African American and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Black or African American and Hispanic or Latino.
White:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both White and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both White and Hispanic or Latino.
More than One Race:
These fields are required.
Enter the expected number of females and males (in the respective fields) who both identify with more than one racial category and are Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who both identify with more than one racial category and are Hispanic or Latino.
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial categories for females, males, and individuals of unknown/not reported sex/gender who are Not Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not reported sex/gender who are Hispanic or Latino. The “Total” fields in the right column will be automatically calculated to total all individuals.
Cumulative (Actual)
Who must complete cumulative (actual) enrollment tables:
You must enter cumulative enrollment counts if your proposed study will use an existing dataset or resource.
For more information about what is considered an existing dataset or resource for inclusion policy, see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the number of females and males (in the respective fields) who are both American Indian/Alaska Native and Not Hispanic or Latino. Enter the number of females and males (in the respective fields) who are both American Indian/Alaska Native and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Asian:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Asian and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Asian and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Black or African American:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Black or African American and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both Black or African American and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
White:
These fields are required.
Enter the number of females and males (in the respective fields) who are both White and Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who are both White and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
More than One Race:
These fields are required.
Enter the number of females and males (in the respective fields) who both identify with more than one racial category and are Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who both identify with more than one racial category and are Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Unknown or Not Reported:
These fields are required.
Enter the number of females, males, and individuals of unknown/not reported sex/gender (in the respective fields) whose race is unknown/not reported and who are Not Hispanic or Latino. Enter the number of females, males, and individuals of unknown/not reported sex/gender (in the respective fields) whose race is unknown/not reported and who are Hispanic or Latino. Enter the number of females, males, and individuals of unknown/not reported sex/gender (in the respective fields) who are both of unknown/not reported race and of unknown/not reported ethnicity. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial categories for females, males, and individuals of unknown/not reported sex/gender who are Not Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not reported sex/gender who are Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown). The “Total” fields in the right column will be automatically calculated to total all individuals.
Non-competing progress report instructions
NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instructional Guide (OMB # 0925-0002, expiration date 01/31/2026)
5.3.6 Editing Inclusion Enrollment Data (OMB #0925-0770, expiration date 09/30/2024)
The following chapter discusses inclusion data in the Human Subjects System (HSS) as accessed and processed via your RPPR. For more information on HSS, please refer to the HSS Online Help or the HSS Training website.
To update inclusion enrollment data, select the Human Subjects link from question G.4 of section G. Special Reporting Requirements. For additional information on inclusion procedures in the RPPR, please review Section G – Special Reporting Requirements.
IMPORTANT: Before selecting the Human Subjects link, click the Save button on the RPPR to save all of your work in Section G. Failure to do so will result in a loss of data on your report.

Figure 27 Human Subjects Link in Question G.4
The Human Subjects link will take you to the Application Information screen. Click on the HSCT Post Submission tab. This will take you to the Study Record(s) screen that displays all the study records and delayed onset records for the grant. You may view the information within a study record, including the last inclusion enrollment report (IER) by clicking on the View button under the Action column within the list of study records.
To edit the inclusion information, click the Edit button at the top of the screen. You will now see an Edit button under the Action column within the list of study records. Click on this Edit button to make changes to the study record information, including the inclusion enrollment data.
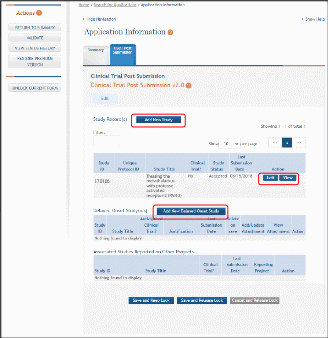
Figure 28 Manage IERs for Single Project
The Inclusion Enrollment Report (IER) is within the Study Record and can be found in Section 2- Study Population Characteristics. Here, you can add a new inclusion enrollment report, or edit a report that was previously created.
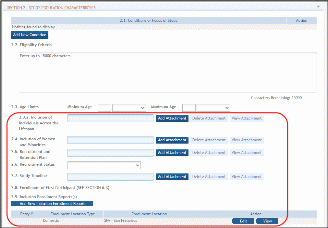
Figure 29 Adding/Editing an Inclusion Enrollment Report
Select the Edit button in the Action column to edit the Inclusion Enrollment Report.
The Inclusion Enrollment Report screen contains the following information:
Inclusion Enrollment Report Fields
Using an Existing Dataset or Resource
Displays Yes/No as indicated by radio buttons
Enrollment Location Type
Displays Domestic/Foreign as indicated by radio buttons
Enrollment Country(ies)
Displays the country of enrollment as identified from a dropdown list
Enrollment Location(s)
Displays a location entered by the user.
Comments
An optional text field for entering cumulative enrollment comments. If any comments for cumulative form were entered before, this field is pre-populated when editing an existing IER.
See the application guide instructions for more information about Inclusion Enrollment report fields.
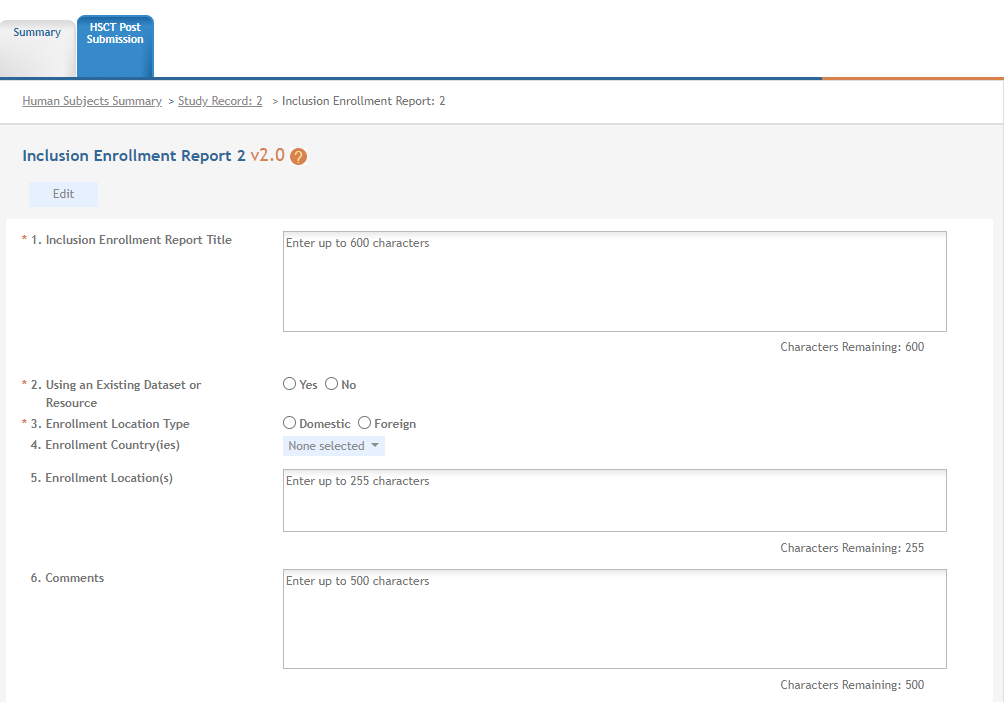
Figure 30 Inclusion Enrollment Report Fields
The cumulative enrollment table includes racial categories along the left side the of the table and ethnic categories, divided by sex/gender, along the top of the table. The individual enrollment count cells are editable and set to zero by default, when populating a new IER. When editing an existing form, these values are pre-populated with any other value previously entered. The total fields are calculated by HSS and sum up as rows and columns accordingly. The total values are not editable fields.
NOTE: The cumulative inclusion table includes fields for entering Unknown/Not Reported race, ethnicity, and sex/gender data.
There are two ways to edit the existing Inclusion Enrollment Report (IER) data for Cumulative (Actual) counts:
You can update the cells online in the existing report itself.
You can download a template for entering participant-level data by clicking on the Download Participant Level Data Template button.
This will download a spreadsheet file in the proper CSV format to be used by the system.
Fill the template out with data, save the changes, and then upload the spreadsheet by clicking on the Upload Participant Level Data Attachment button. This uploaded data will populate the cells in the report.
You can click on the Download Current Participant Level Data button to download the file containing the data for your own records.
If you need to clear the current records, use the Remove Current Participant Level Data button.
Update the values in the individual enrollment count cells as necessary, and select the Save and Release Lock button. To leave the form without saving any changes, select the Cancel and Release Lock button instead. Saving and canceling both return you to the Study Record(s) screen.
POLICY: Individual-level participant data on sex/gender, race, ethnicity and age at enrollment is required in progress reports for competitive applications submitted for due dates on or after January 25, 2019 (See NIH Guide Notice NOT-OD-18-116). Inclusion data for subsequent RPPRs must be provided using the file upload option.
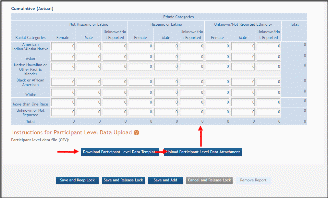
Figure 31 Cumulative (Actual) Enrollment Table
5.2.4.1 Changes to Planned Enrollment
If there are changes from the planned enrollment originally approved for funding, contact the program officer to discuss updating/revising the planned enrollment. See Section G – Special Reporting Requirements of this guide for more information.
From the Study Record(s) screen, select the Edit link in the Action column of the Inclusion Enrollment Report section to edit the Inclusion Enrollment Report.
The planned enrollment table includes racial categories along the left side of the table and ethnic categories, divided by sex/gender, along the top of the table. The individual enrollment count cells are editable and set to zero by default, when populating a new IER. When editing an existing form, these values are pre-populated with any other value previously entered. The total fields are calculated by HSS and sum up as rows and columns accordingly. The total values are not editable fields.
POLICY: For additional information on racial and ethnic categories or inclusion policy, refer to Inclusion of Women and Minorities as Participants in Research Involving Human Subjects and Inclusion - Basis of Sex/Gender and Race/Ethnicity.
Update the values in the individual enrollment count cells as necessary, and select the Save and Release Lock button. To leave the form without saving any changes, select the Cancel and Release Lock button instead. Saving and cancelling both return you to the Study Record(s) screen.
5.2.4.2 No Inclusion Data Records Provided
When inclusion monitoring is required and no IERs exist, RPPR system will NOT allow the submission of the progress report without IER(s). For the current FY it will display an error message and require that you submit a new enrollment record.
This is true for the current FY of a multi-year award as well. For the past FYs (when the progress report is late), a standard message is displayed in lieu of the error message as follows:
NIH policy requires inclusion to be monitored, but no inclusion data record(s) (IERs) have been provided.
This standard message will appear on both the screen and the PDF version of the progress report.
Submit New Planned Inclusion Record
From the Study Records(s) screen, select the Add New Inclusion Enrollment Report to create a new IER. If no study records exist, first add a study record using instructions in the online help.
Upon a successful save of a new IER, attributes (Foreign/domestic indicator/planned comments), Planned Inclusion Data (as entered), and Cumulative Inclusion Data (as zeros) are also created; the new IER is assigned a unique IER #.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | OPERA DGSI |
| File Modified | 0000-00-00 |
| File Created | 2024-09-28 |
© 2026 OMB.report | Privacy Policy