Incentives Pilot Proposal
Appendix U - Incentives Pilot Proposal - New.docx
[ATSDR] National Amyotrophic Lateral Sclerosis (ALS) Registry
Incentives Pilot Proposal
OMB: 0923-0041
Proposal for the CDC/ATSDR National ALS Registry Incentives Pilot Project
Background:
Amyotrophic Lateral Sclerosis, or ALS, is a progressive neurodegenerative, fatal disease without an effective treatment or cure, with most patients dying 2-5 years after receiving their diagnosis (1). As mandated by Congress, the goal of the National Amyotrophic Lateral Sclerosis (ALS) Registry study is to better describe the incidence and prevalence of ALS and to identify risk factors for the disease (2). The National ALS Registry (Registry) is a program to collect, manage and analyze data about persons living with ALS. It includes a two-prong approach using data from existing national administrative databases along with web portal data provided by persons with ALS who choose to register and complete risk factor surveys (3,4). Researchers can use Registry data to look for disease pattern changes over time and try to identify whether there are common risk factors. The information collected is used to: 1) estimate the number of new cases of ALS diagnosed each year; 2) estimate the number of people who have ALS at any given point in time; 3) better understand who gets ALS and what factors affect the disease; and 4) enhance research that could improve care for people with ALS.
The Agency for Toxic Substance and Disease Registry (ATSDR) endeavors to improve the completeness, representativeness, and accuracy of the Registry data over time. Enrollment rates for those self-enrolling in the Registry have been lower than expected based on census and prevalence data estimates. Thus, a small incentive will help increase enrollment and participation in the risk factor surveys.
Background Data
Every month, the Registry measures the rate of enrollment within the self-enroll portal across various states and categorizes the states into three tiers. ATSDR generates this table monthly to compare the percent of persons with ALS who have registered in the Registry for each state to the percent registered for the U.S. These data are provided as one measure of registration performance and shared internally with our Partners to promote increased education and awareness about the Registry amongst these states. Historically, the state of Texas has been among the under-performing states in the Tier 3 category, meaning it consistently has been enrolling less than the national average.
In the last 2 years (2022 and 2023), 1,728 patients living with ALS enrolled in the Registry. Of those who enrolled, 356 (20.6%) completed 1-9 surveys, while 372 (21.5%) completed 10-17 surveys, and 195 (11.3%) participants completed all 18 surveys. Additionally, since 2018, there has been a gradual decline in registrations in the Registry across the U.S. (Figure 1). Comparatively, of the 84 participants who enrolled in the Registry from the state of Texas in 2022 and 2023, 15 (17.9%) completed 1-9 surveys, 21 (25%) completed 9-17 surveys, and only 11 (13.1%) completed all 18 surveys. Furthermore, Texas has historically fluctuated in enrollment in the Registry (Figure 2) and would serve as an ideal candidate for the incentive pilot project.
Figure 1. Annual Enrollment for the National ALS Registry for 2018-2023 across the U.S.
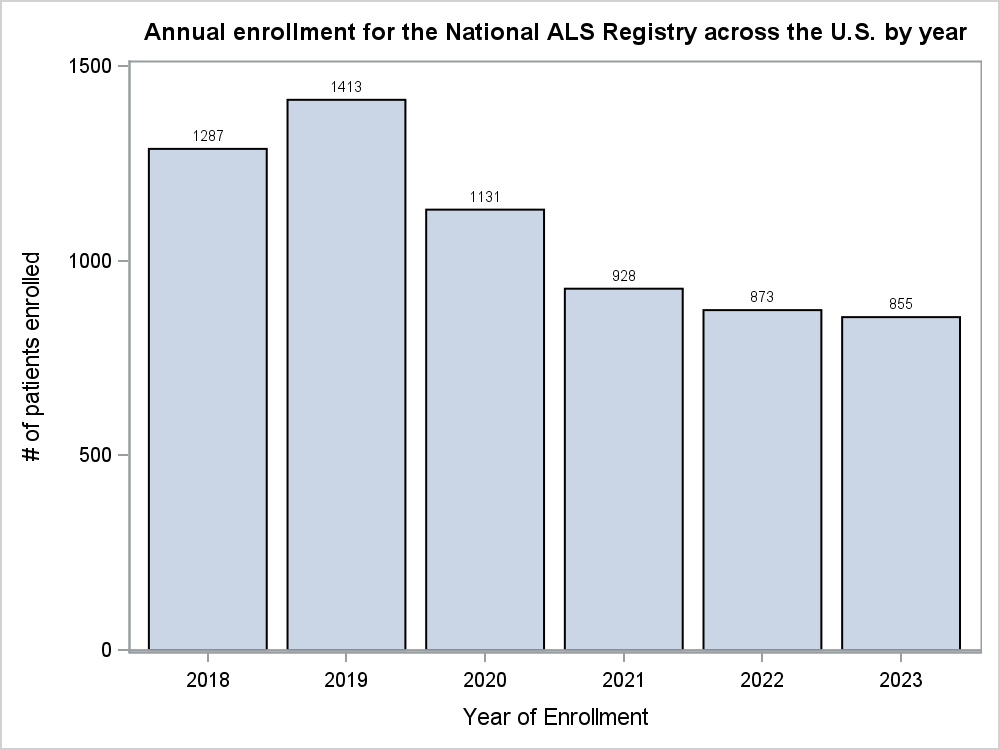
Figure 2. Annual Enrollment for the National ALS Registry in Texas for 2019-2023
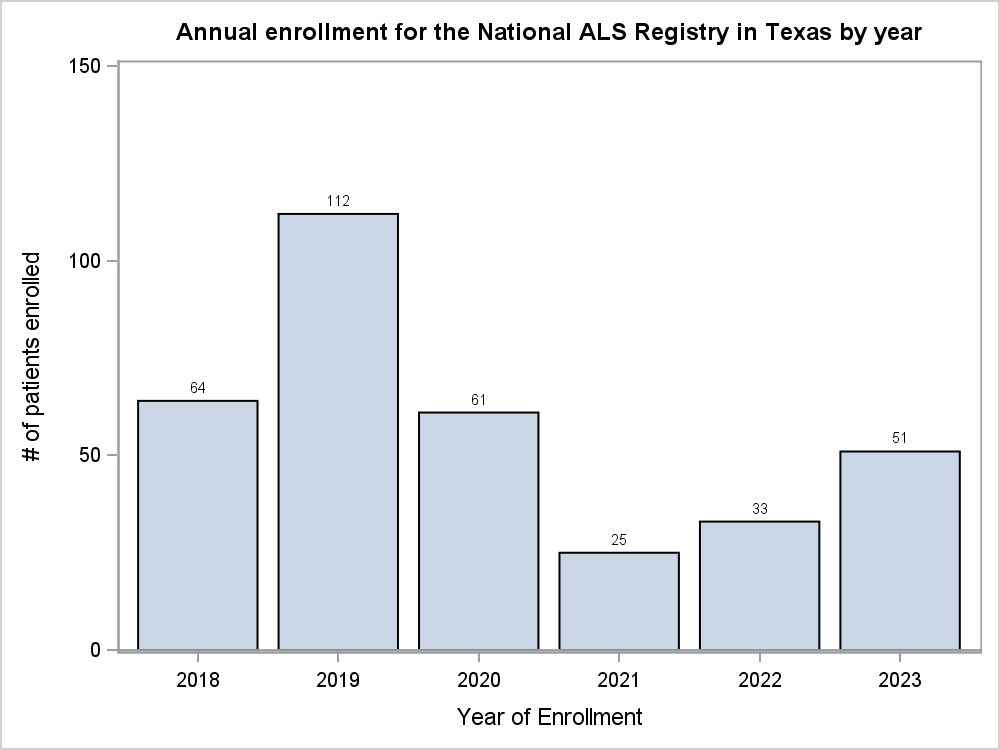
Finally, the Texas Chapter of the ALS Association serves a total of 1,050 people living with ALS each month. The mission of the ALS Association is to discover treatments and a cure for ALS, and to serve, advocate for, and empower people affected by ALS to live their lives to the fullest. Among the 13 multidisciplinary clinics in Texas, an average of 37 people living with ALS register with the ALS Association each month. This shows that a collaboration with the Registry and ALS Association would improve outreach to those living with ALS and in turn increase enrollment and survey completion for the Registry.
Goal
The goal of the incentive pilot project will be measured in two parts. The first goal is to increase participation in the Registry. Using the baseline registration rates for the state of Texas in 2022 and 2023, the rates of registration for the pilot will be compared to these rates to measure improvement in enrollment rates. The second goal is to improve risk factor survey completion rates for all 18 surveys. Because the incentive will be provided in two parts, this will incentivize individuals who have registered to also complete risk factor surveys. In addition to increased percentage of enrollment and survey completion, the goal of the pilot will be to enroll at least 100 persons living with ALS in Texas for the duration of the pilot (6-9 months).
Summary and Explanation of Justifications:
Below are some justifications and rationale for the development and implementation of this incentive pilot project.
People diagnosed with ALS are dealing with a serious, debilitating illness that makes it challenging to prioritize participating in research such as the Registry.
ALS is a disease that takes a significant physical, financial, social, and emotional toll on the patient, caregiver, and family. Patients who want to participate in research such as the Registry are often overwhelmed and face daily challenges. Enrollment in the Registry can offer additional resources to those living with ALS that can help to manage or cope with the disease and access to up-to-date info from ALS research. The incentive to provide a monetary incentive is to increase the number of patients living with ALS and their access to resources provided by the Registry.
Physical decline and mobility challenges – ALS causes a progressive decline in motor function, leading to muscle weakness, stiffness, and eventually paralysis. The physical impacts of ALS require durable medical equipment such as a rehab style power wheelchair, ramps, patient lift, hospital bed, and other adaptive equipment.
Communication and swallowing challenges – ALS affects the muscles that are responsible for speaking and swallowing. People with ALS often lose the ability to speak and may require assistive or alternative communication devices to share information with others. They may also require a modified diet to avoid choking or a feeding tube for nutrition and medication.
Breathing difficulties – ALS affects the muscles that are responsible for breathing. Most people with ALS require a breathing machine such as a non-invasive ventilator or a tracheostomy.
Emotional, psychological, and financial stress – Coping with the knowledge of a progressive, fatal disease leads to significant psychological and emotional distress. In addition, the cost of equipment, home modifications, caregivers, and medical care often cause additional stress. Many spouses, parents, and adult children quit working to care for their loved one. Caregivers are known to experience high levels of stress.
Caregiving, home care, and multidisciplinary care – The standard of care for a person with ALS is to attend a half-day appointment at a multi-disciplinary ALS clinic every three months. The clinic is often 30 minutes to several hours away by car. Home care appointments, outpatient visits, and allied health professional services (physical therapist, occupational therapist, respiratory therapist, registered dietician, speech and language pathologist, social worker, counseling) also add demands to the time and energy of a person with ALS and their caregiver.
The Registry enrollment and completion of risk factor surveys are lower than expected based on census and prevalence data. ALS is also a non-notifiable disease in the United States, which is an additional challenge to enrollment. Because the Registry is the only congressionally mandated program determining ALS prevalence and incidence, it is important that those living with ALS self-enroll in the Registry and complete the risk factor surveys.
The
National ALS Registry is central to disease surveillance and research
in ALS and declining registrations and survey completions will
eventually jeopardize needed advancements in preventing and treating
ALS. In 2019, pre-COVID data, showed 1,413 persons living with ALS
enrolled in the Registry. In 2020, the number fell to 1,131. In
2023, the number of persons living with ALS enrolling in the Registry
was just 855. The number of completed surveys is down a similar
amount as well with fewer enrolled patients. When people do
successfully enroll, they often do not complete all the risk factor
surveys. Registration into the Registry and the completion of risk
factor surveys is crucial to understanding ALS as a disease and how
it impacts those across the United States.
The COVID-19 pandemic has had a negative impact on awareness and participation in the Registry.
The
COVID-19 pandemic resulted in reduced contact between patients and
clinic staff, support group facilitators, and care managers. The
constant communication and contact with these individuals is crucial
for ALS patients. These are the very interactions that result in
patients learning about the Registry and its benefits. It is believed
that the pandemic is one factor contributing to recent declines in
enrollment. The incentive program will help to reinvigorate interest
and participation in the Registry.
The Registry’s risk factor surveys can take a substantial amount of time to complete, and a $150 token of appreciation is an appropriate compensation in recognition of the effort required for people living with ALS.
Compared to enrolling in the Registry, which can take a few minutes, completing all the risk factor surveys has higher burden hours for completion for the person living with ALS. Thus, a monetary incentive recognizes this effort and provides an incentive for completion. A $150 token of appreciation also reflects the added effort to complete enrollment and 18 surveys for someone with ALS who faces mobility, communication, and other challenges that can make research participation difficult. Because ALS is a non-notifiable disease, the enrollment and survey completion adds to the incidence and prevalence data, along with risk factor information available for ALS researchers.
Summary of other Federal incentives research/programs
Below is a list of other federal incentive programs across CDC/ATSDR and the federal government who have successfully used monetary incentives to increase enrollment and participation. The data below of previous programs indicates a higher response and retention rate for those who were provided incentives versus no incentives. This provides a justification for the benefit and use of incentive programs to assist with enrollment and survey completion.
Behavioral and Clinical Characteristics of Persons with Diagnosed HIV Infection—Medical Monitoring Project, United States 2021 Cycle (June 2021–May 2022)
The Medical Monitoring Project reports health statistics on adults diagnosed with HIV. The organization that sponsored/hosted this research is the Centers for Disease Control and Prevention’s Division of HIV Prevention. This iteration of the study occurred from June 1, 2021 to May 31, 2022 and included participants sampled from 16 states (5). Participants were given $50 in cash or given equivalent compensation (such as gift cards) for participation. Out of the 9,700 people who were sampled from National HIV Surveillance System, 3,955 participated (44% response rate).
Clearing the Air: Smoke-Free Housing Policies, Smoking, and Secondhand Smoke Exposure Among Affordable Housing Residents in Minnesota, 2014–2015
Researchers examined the effect of smoke-free policies on smoking rates and exposure to secondhand smoke among residents in affordable housing in Minnesota. The organization that sponsored/hosted this research is the Statewide Health Improvement Program and the Centers for Disease Control and Prevention. Participants were offered a $15 gift card and $20 gift card for completing Time 1 and Time 2 survey, respectively (6). Time 1 survey was distributed to all residents one month before implementing the smoke-free policy (February 2014 – March 2015). Time 2 survey was distributed to remaining residents 6 months after the policy implementation (September 2014 October 2015). Out of 578 residents, 289 completed Time 1 survey, resulting in a 50% response rate. Out of 264 residents, 180 completed Time 2 survey, resulting in a 68.2% response rate and 62.3% retention rate.
Hypertension-Focused Medication Therapy Management: A Collaborative Pilot Program Uniting Pharmacists, Public Health, and Health Insurers in Wisconsin
The Wisconsin Department of Health Services’ Division of Public health, Pharmacy Society of Wisconsin, and NeuGen partnered together to create a pilot program that aimed to increase patient knowledge and beliefs about hypertension, use of blood pressure self-management tools, and decrease barriers to medication adherence (7). The organization that sponsored/hosted the research was the Centers for Disease Control and Prevention’s Division for Heart Disease and Stroke Prevention. The study was conducted between December 2017 and September 2018. Eligible NeuGen members were offered a $25 gift card to a local gas station or convenience store after completing 2 in-person visits with the pharmacist. Out of the 145 eligible members, 61 of them participated in the pilot program (42% participation rate).
Comparing Incentives to Increase Response Rates Among African Americans in the Ohio Pregnancy Risk Assessment Monitoring System
Researchers for the Ohio Pregnancy Risk Assessment Monitoring System (PRAMS) sought to determine the effect of a $10 CVS gift card incentive on the response rate from African American women compared to the 30 min prepaid phone card that is typically given for participation (8). The study was conducted from June to November 2008 and participants were randomly assigned to either the experimental or control group. Incentives were given after partial or full completion of their first survey. Overall, the response rate for the experimental group was 60.5%, while the response rate for the control group was 48.5%
Randomized Control Trial of Unconditional versus Conditional Incentives to Increase Study Enrollment Rates in Participants at Increased Risk of Lung Cancer
The organization that sponsored/hosted this research was the National Institutes of Health (NIH). The researchers sought to determine the impact of unconditional vs conditional incentives on study enrollment rates among participants completing lung cancer screening. Unconditional incentives refer to incentives given regardless of survey completion while conditional incentives refer to incentives given after survey completion. The study was conducted from January 2018 to December 2018 and a $20 gift card was given as the incentive (9). 112 participants were randomly assigned to the unconditional incentive group, while 111 were randomly assigned to the conditional incentive group. Participants in the unconditional incentive group had an enrollment rate of 54.5% while those in the conditional incentive group had a rate of 41.4%. Using multivariable logistic regression, they also found that participants in the unconditional incentive group were 74% more likely to enroll in the study compared to those in the conditional incentive group.
Gift Card Incentives and Non-Response Bias in a Survey of Vaccine Providers: The Role of Geographic and Demographic Factors
Researchers with the Washington State Health Department evaluated the role of gift card incentives in non-response bias in a survey among Washington H1N1 influenza vaccine providers (10). The study occurred from 2009 to 2010 and included a random sample of 765 providers. Providers were given a $25 Target gift card in their survey kit. Of the 765 providers, 619 returned their surveys, resulting in a response rate of 80.9%.
Target Audience
Individuals with a current ALS diagnosis, who are currently eligible but not yet enrolled in the Registry. To enroll in the Registry, individuals must be US citizens or legal residents with an ALS diagnosis. Since this is a pilot project, this project’s demographic target audience will be those ALS patients residing in the state of Texas, who have enrolled with the Texas ALS Association.
Inclusion and Exclusion Criteria:
Inclusion Criteria:
Registered with The Texas ALS Association.
Diagnosis of ALS.
Already enrolled in the Registry and has not completed all the risk factor surveys.
U.S. citizen or legal resident who lives in Texas.
Person who elects to participate within the designated time-period for the pilot.
Exclusion Criteria:
Diagnosis of non-ALS disease such as Primary Lateral Sclerosis (PLS).
Deceased persons.
Already enrolled in the Registry and completed all the risk factor surveys.
Persons not willing to provide an email address or consent to enroll in the Registry.
Person who is interested in signing up for the pilot after the end of the pilot period.
Incentives Amount
The National ALS Registry proposes one primary option for incentive distribution to those participating in the pilot project. Also included is a secondary option for incentive distribution.
Option A (primary option): The enrollee will receive $50 for enrolling in the National ALS Registry and second $100 for completing all applicable 18 risk factor surveys. Both rewards will be distributed separately, upon completion of the task.
Option B: The enrollee will receive $25 for enrolling in the National ALS Registry and second $100 for completing all applicable 18 risk factor surveys.
Project parameters/timeline
This project will be conducted on a six-to-nine-month timeline (pending OMB approval). Once OMB approval is received, the pilot will be launched in the state of Texas. The ALS Association will be the key collaborator for the pilot and will promote and communicate the launch of this pilot through care services, events, clinic, and direct messages with The ALS Association’s Texas Care Services team.
Tentative Pilot Program Timeline
 *Dates
in the table are included as an example. Actual dates may vary.
*Dates
in the table are included as an example. Actual dates may vary.
Incentives
Plan
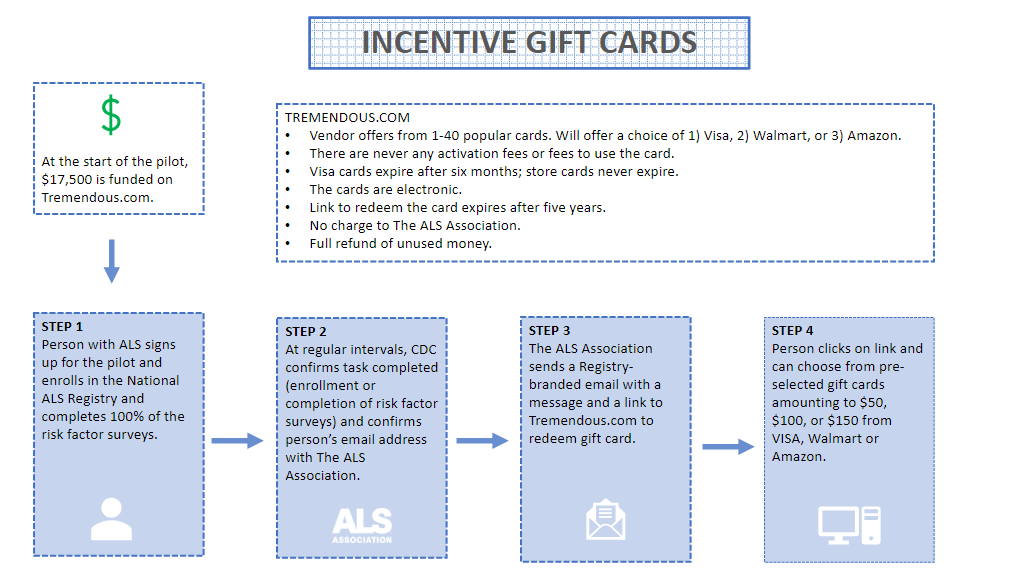
Incentive Enrollment Eligibility and Process

Marketing Outreach – Texas Chapter (ALS Association)
Training for chapter staff – The ALS Association will administer a virtual one-hour, comprehensive training on the National ALS Registry and the incentive pilot program parameters and protocols to Care Services staff in Texas.
Resources for recruitment – Up to 100-200 participants will be recruited by Care Services staff engaging with people with ALS at clinic, Walk to Defeat ALS events, support groups, email, and phone calls. Messaging will be adhered to existing and approved Registry flyers and scripts.
Spanish-speaking participants – People with ALS who are Spanish-speaking or speak English as a second language will be informed that the Registry has a Spanish version at cdc.gov/ela.
Eligibility Verification
On a weekly basis, one single point of contact from ATSDR/ Registry team and one individual from the ALS Association will confirm individuals who have 1) enrolled and those who have 2) completed the risk factor surveys. This confirmation will be over the phone and will prevent the sharing of data through email. This regular confirmation will ensure all patients enrolling in the incentive program to be accounted for. Additionally, a reminder email will be sent out to participants who have enrolled but have not completed all the risk factor surveys.
Enrollment Eligibility Form
An online enrollment form will be required to join the pilot. The form can be completed by the person living with ALS or by an ALS Association Care Services staff member on the patient’s behalf. The enrollment form will outline the pilot program parameters including:
Attest that they meet the criteria to participate.
List the name, address, and email that will be used for enrolling in the Registry.
Check the appropriate Care Services staff contact from a list.
Requirements to qualify for the incentive.
Email Registryinfo@als.org and copy Care Services staff when enrollment is complete to qualify for a gift card of $50.
Email Registryinfo@als.org and copy Care Services staff when risk factor surveys have been completed to qualify for a gift card of $100.
OREmail Registryinfo@als.org and copy Care Services staff when both enrollment and risk factor surveys have been completed for a gift card of $150.
Incentive Project Sign Up Form
The following form will be used to enroll participants into the Incentives Pilot Project. Because national projections are not being met for enrollment and survey completion, this strategy will be implemented in hopes to increase Registry enrollment and risk factor survey completion. This form will not change any burden hours (OMB), as we are not currently meeting our anticipated enrollment.


Incentive Distribution/ Budget
The monetary incentive will be distributed using an electronic gift card using a third-party vendor. The email (template to be included) with the electronic gift card will be distributed on behalf of the National ALS Registry (not the ALS Association). The contract funds from FY’25 will be used to fund the pilot incentive project. Contract funds will not fund a nationally available incentive or expanded rollout of the pilot. To ensure fraud protection, the gift card will be emailed to the same email address used to enroll in the National ALS Registry.

Sample
Email Template

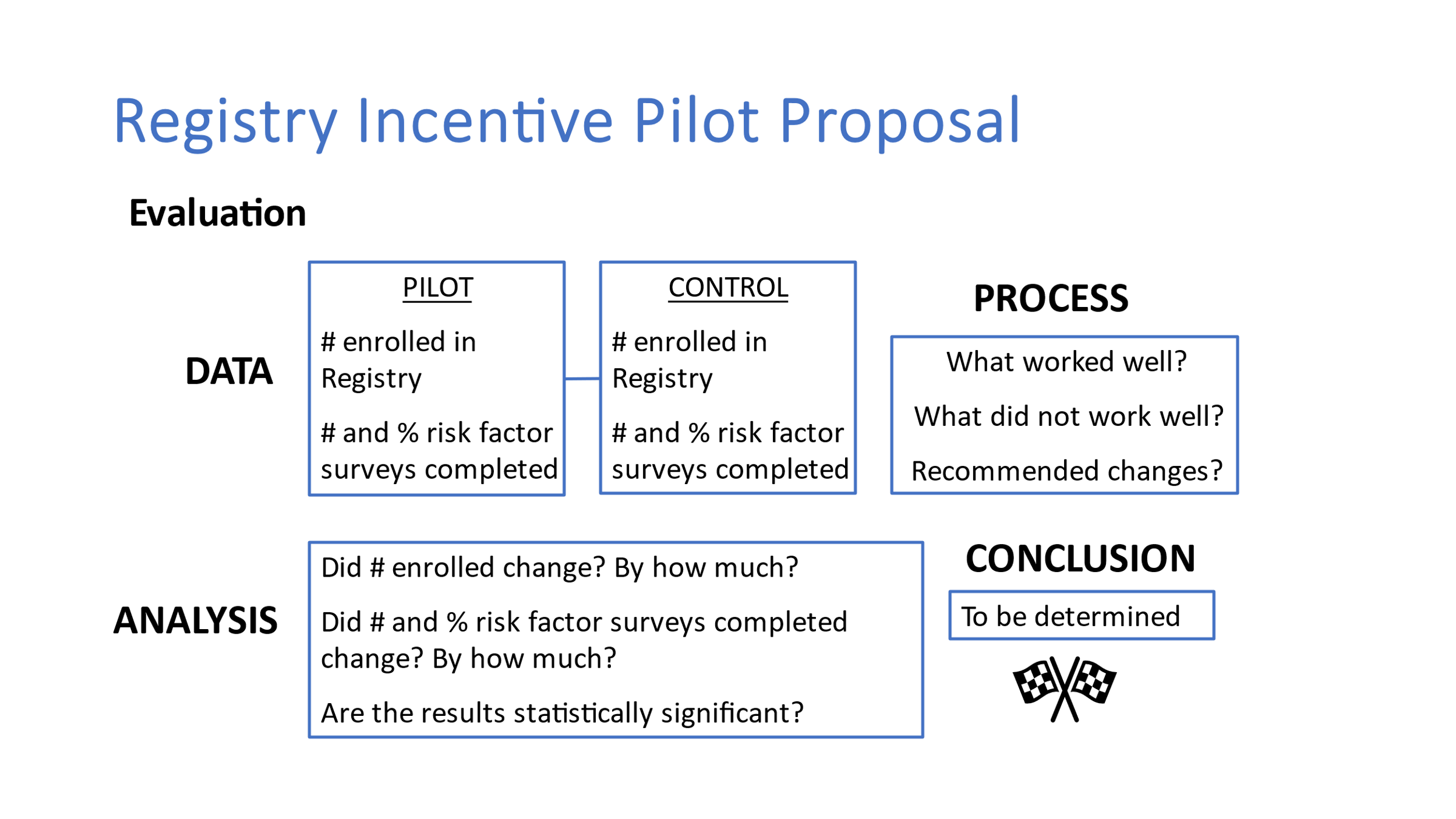
Program Evaluation
Evaluation metrics will include:
How many people enrolled in the Registry in the US.
How many people enrolled in the Registry in Texas in a previous 6-9 month time period
How many people completed risk factor surveys in a previous 6-9 month time period
Percent change/increase in enrollment
Percent change/increase in survey completion
Statistical differences in enrollment and survey completion within the state as well as across the US
References
Mitsumoto H, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia, PA: F.A.D. Company, Editor; 1998.
US Public Health Service. ALS registry act. Washington, DC: 110th Congress; 2008.
Mehta P, Raymond J , Zhang Y, Punjani R, Han M, Larson T, Muravov O, Robert H. Lyles & D. Kevin Horton (2023) Prevalence of amyotrophic lateral sclerosis in the United States, 2018, Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 24:7-8, 702-708, DOI: 10.1080/21678421.2023.2245858
Mehta P, Raymond J, Punjani R, Larson T, Han M, Bove F, et al. Incidence of amyotrophic lateral sclerosis in the United States, 2014-2016. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23:378–82.
Behavioral and Clinical Characteristics of Persons with Diagnosed HIV Infection—Medical Monitoring Project, United States 2021 Cycle (June 2021–May 2022). 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance-special-reports/no-32/index.html
Kingsbury JH, Reckinger D. Clearing the Air: Smoke-Free Housing Policies, Smoking, and Secondhand Smoke Exposure Among Affordable Housing Residents in Minnesota, 2014–2015. Prev Chronic Dis 2016;13:160195. DOI: http://dx.doi.org/10.5888/pcd13.160195
Thompson H, Swander L, Cohen R, Lukazewski A, Bartholow T, Pesik M, et al. Hypertension-Focused Medication Therapy Management: A Collaborative Pilot Program Uniting Pharmacists, Public Health, and Health Insurers in Wisconsin. Prev Chronic Dis 2020;17:200058. DOI: http://dx.doi.org/10.5888/pcd17.200058external icon
Liu ST, Geidenberger C. Comparing incentives to increase response rates among African Americans in the Ohio pregnancy risk assessment monitoring system. Matern Child Health J. 2011;15(4):527-533. doi:10.1007/s10995-010-0609-4
Kumar AD, Durham DD, Lane L, Perera P, Rivera MP, Henderson LM. Randomized control trial of unconditional versus conditional incentives to increase study enrollment rates in participants at increased risk of lung cancer. J Clin Epidemiol. 2022;141:11-17. doi:10.1016/j.jclinepi.2021.08.027
Van Otterloo J, Richards JL, Seib K, Weiss P, Omer SB. Gift card incentives and non-response bias in a survey of vaccine providers: the role of geographic and demographic factors. PLoS One. 2011;6(11):e28108. doi:10.1371/journal.pone.0028108
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Berry, Jasmine (ATSDR/OAD/OIA) |
| File Modified | 0000-00-00 |
| File Created | 2024-09-05 |
© 2026 OMB.report | Privacy Policy