GFI Quant Info 2023
Prescription Drug Advertisements and Product Communications
GFI Quant Info 2023
OMB: 0910-0686

Presenting Quantitative Efficacy and Risk Information in
Direct-to-Consumer (DTC) Promotional Labeling and Advertisements
Guidance for Industry
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Center for Veterinary Medicine (CVM)
[Date]
Advertising
OMB Control Number 0910-0686
Expiration Date: 08/31/2024
See additional PRA statement in section IV of this guidance.
Presenting Quantitative Efficacy and Risk Information in
Direct-to-Consumer (DTC) Promotional Labeling and Advertisements
Guidance for Industry
Additional copies are available from:
Office of Communications, Division of Drug Information
Center for Drug Evaluation and Research
Food and Drug Administration
10001 New Hampshire Ave., Hillandale Bldg., 4th Floor
Silver Spring, MD 20993-0002
Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353
Email: druginfo@fda.hhs.gov
https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs
and/or
Office of Communication, Outreach and Development
Center for Biologics Evaluation and Research
Food and Drug Administration
10903 New Hampshire Ave., Bldg. 71, Room 3128
Silver Spring, MD 20993-0002
Phone: 800-835-4709 or 240-402-8010
Email: ocod@fda.hhs.gov
and/or
Policy and Regulations Staff, HFV-6
Center for Veterinary Medicine
Food and Drug Administration
7500 Standish Place, Rockville, MD 20855
https://www.fda.gov/animal-veterinary/guidance-regulations/guidance-industry
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Center for Veterinary Medicine (CVM)
[Date]
Advertising
TABLE OF CONTENTS
I. INTRODUCTION 1
II. BACKGROUND 2
III. RECOMMENDATIONS FOR PRESENTING QUANTITATIVE EFFICACY AND RISK INFORMATION IN DIRECT-TO-CONSUMER PROMOTIONAL LABELING AND ADVERTISEMENTS 3
A. Quantitative Efficacy or Risk Information From the Control Group 3
B. Probability Presentations 4
1. Absolute Frequencies and Percentages 4
2. Relative Frequencies 5
C. Formatting Quantitative Efficacy or Risk Information 6
D. Visual Aids 8
IV. PaperWORk REDUCTIon Act of 1995 9
References 11
Presenting Quantitative Efficacy and Risk Information in Direct-to- Consumer (DTC) Promotional Labeling and Advertisements
Guidance for Industry1
This guidance represents the current thinking of the Food and Drug Administration (FDA or Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the FDA office responsible for this guidance as listed on the title page.
I. INTRODUCTION
This guidance provides recommendations for presenting quantitative efficacy and risk information2 in direct-to-consumer (DTC) promotional labeling and advertisements for prescription human drug and biological products and prescription animal drugs and in DTC promotional labeling for over-the-counter animal drugs3 (collectively, promotional communications).4 For the purposes of this guidance, quantitative efficacy and risk information refers to information that numerically addresses the likelihood or magnitude of a drug’s efficacy or risks.
The guidance outlines FDA’s recommendations for how firms5 that include quantitative efficacy or risk information in DTC promotional communications for their drugs can make the language and presentation more consumer-friendly.6 The guidance covers the following topics:
Providing quantitative efficacy or risk information for the control group, when applicable
Presenting probability information in terms of absolute frequencies, percentages, and relative frequencies
Formatting quantitative efficacy or risk information
Using visual aids to illustrate quantitative efficacy or risk information
In general, FDA’s guidance documents do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
II. BACKGROUND
Under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and FDA’s implementing regulations, drug promotional labeling and prescription drug advertising must be truthful and non-misleading, convey information about the drug’s efficacy and its risks in a balanced manner, and reveal material facts about the drug.7 Firms generally have flexibility with respect to the presentation of efficacy and risk information about their products as long as the presentation is not false or misleading and complies with other applicable statutory and regulatory requirements. When firms develop DTC promotional communications, they should consider how to best convey information about a drug’s efficacy and risks so the audience understands the information. This includes consideration of whether to provide efficacy and risk information by using words, numbers, or visual aids, or a combination of these elements.
FDA has observed an increase in quantitative presentations of efficacy and risk information in DTC promotional communications submitted to the Agency. Research on the communication of treatment information suggests that consumers can recall and comprehend efficacy and risk information when it is provided quantitatively (Buchter et al. 2014; O’Donoghue et al. 2014b; Schwartz et al. 2007; Schwartz et al. 2009; Sullivan et al. 2015; Sullivan et al. 2019; Trevena et al. 2013; West et al. 2013; Woloshin et al. 2004). When compared to qualitative descriptions of efficacy and risk information, quantitative information can improve consumers’ accuracy in estimating the drug’s benefits and risks (Sullivan et al. 2015; West et al. 2013). This is due in part to how consumers differ in their interpretations of qualitative descriptors (e.g., rare, common, most) and how the context in which qualitative terms are presented can affect how consumers understand them (Buchter et al. 2014; Fagerlin et al. 2007; Lipkus 2007; Visschers et al. 2009). Quantitative efficacy or risk information may offer more precision than qualitative information; therefore, consumers can use quantitative efficacy and risk information to form more accurate perceptions about the drug (Lipkus 2007).
DTC promotional communications containing quantitative efficacy or risk information should be accurate and understandable. FDA recognizes that firms may experience challenges when determining how to present this kind of quantitative information in DTC promotional communications. For these reasons, FDA is issuing this guidance to provide recommendations for presenting quantitative efficacy and risk information in DTC promotional communications and to encourage firms to follow these recommendations when including such information in their DTC promotional communications.
The recommendations in this guidance generally apply to quantitative efficacy and risk presentations in DTC promotional communications across various media types (e.g., print, electronic, audiovisual). Firms should consider the amount of space or time available and any other factors specific to the media type in which their presentation will appear when determining how to present quantitative efficacy or risk information in their DTC promotional communications so that consumers have an opportunity to attend to and understand it.
The examples in this guidance are intended to illustrate recommended approaches to presenting quantitative efficacy and risk information in DTC promotional communications. Each example is meant to address a specific concept described in the guidance; a given example may not illustrate every recommendation outlined. The examples do not encompass every potential promotional scenario or consideration and do not necessarily reflect an evaluation of a complete promotional piece, including whether the piece complies with other applicable requirements. All recommendations discussed in this guidance should be taken into consideration even if not expressly illustrated in an example.
III. RECOMMENDATIONS FOR PRESENTING QUANTITATIVE EFFICACY AND RISK INFORMATION IN DIRECT-TO-CONSUMER PROMOTIONAL LABELING AND ADVERTISEMENTS
A. Quantitative Efficacy or Risk Information From the Control Group
When a study includes a control group, firms that provide quantitative efficacy or risk information about a drug in DTC promotional communications should provide quantitative information from both the treatment group and the relevant control group. Information from the control group plays an important role in evaluating a drug’s benefits and risks (O’Donoghue et al. 2014a). Including quantitative benefit or risk measures observed in the control group when providing corresponding quantitative measures observed in the treatment group improves consumers’ ability to process and comprehend the drug’s benefits and risks and can lead to more informed decision making (O’Donoghue et al. 2014a; Schwartz et al. 2009). Research suggests that consumers can use the information about the control group to form accurate perceptions about a drug’s benefits (including efficacy) and risks (O’Donoghue et al. 2014a; Schwartz et al. 2009; Sullivan et al. 2013). Promotional communications that include control group information should accurately describe the comparator used in the control group.
Example8 1: In a clinical trial of 173 participants, 68% of patients who were treated with Drug X plus a sulfonylurea experienced a reduction in blood glucose levels, while 33% of patients treated with a sulfonylurea alone experienced a reduction in blood glucose levels.
The firm is developing a social media web page for Drug X and includes a presentation that 68% of patients treated with Drug X plus a sulfonylurea experienced a reduction in blood glucose levels.
To improve consumers’ ability to comprehend the drug’s effect on blood glucose levels, the firm should also include that 33% of patients treated with a sulfonylurea alone experienced a reduction in blood glucose levels.
B. Probability Presentations
Firms should consider the following recommendations when presenting quantitative probability information about their drug’s efficacy and risks.
1. Absolute Frequencies and Percentages
Firms presenting quantitative efficacy or risk probabilities in DTC promotional communications should convey the information in terms of absolute frequencies (e.g., 57 out of 100) or percentages (57%). Research suggests that using these formats to express probabilities when communicating health information can improve consumers’ comprehension and ability to recall the information (Lipkus 2007; Zipkin et al. 2014). Additionally, consumers receiving information about a drug’s efficacy and risk rates in terms of absolute frequencies or percentages can more easily process and evaluate the information than when the same information is in a format that requires them to perform a calculation to interpret the probabilities (Lipkus 2007; O’Donoghue et al. 2014b; Sullivan et al. 2015).
Example 2: A firm is developing a magazine advertisement and includes a presentation showing that in clinical trials, most patients experienced a response after
12 weeks of treatment with Drug X.
The firm wants to add numeric values to the presentation to help consumers understand this information.
To communicate this information in a manner that will facilitate consumer comprehension, the firm presents the information as an absolute frequency: In a clinical trial, 78 out of 100 patients experienced a response after 12 weeks of treatment with Drug X, compared to 20 out of 100 patients on placebo.
Example 3: A firm plans to include quantitative information in a patient mailer for Drug X about the most common adverse reaction reported in its clinical trial that compared Drug X to Drug Y: nausea.
To allow consumers to easily process this information, the firm presents the information as a percentage: In a clinical trial, 45% of patients experienced nausea during 20 weeks of treatment with Drug X, compared to 18% of patients during treatment with Drug Y.
2. Relative Frequencies
Research suggests that consumers do not understand relative frequencies (e.g., 33% reduction in symptoms; 3 times as likely to experience a side effect) in health communications as easily as they understand other formats for presenting probabilities, such as absolute frequencies or percentages (Covey 2007; Fagerlin et al. 2007; Zipkin et al. 2014). Consumers may also find the efficacy or risk probability described as a relative frequency harder to comprehend and more favorable as compared to the absolute frequency, which could lead to consumers’ over- or underestimating how well the drug works or the magnitude of the risk associated with the drug (Ancker et al. 2006; Covey 2007; Zipkin et al. 2014).
If firms choose to present efficacy or risk probabilities as relative frequencies, they should add context to the relative frequency presentation to improve consumers’ ability to accurately understand the efficacy or risk information. Specifically, firms should include the corresponding absolute probability measures in presentations of relative frequency measures to provide the information in a way that does not require further calculation about the effect being communicated (Covey 2007; O’Donoghue et al. 2014b; Sullivan et al. 2015). Firms should present the absolute probability measure prominently and in direct conjunction with the relative frequency measure.
Example 4: A firm is developing a DTC television advertisement for Drug X, which is indicated to reduce the risk of stroke. In a clinical trial, the following absolute risk reductions were observed: 1% of patients treated with Drug X had a stroke, compared to 2% of patients in the control group. This represents a 50% relative reduction in risk of stroke.
The firm wants to include this information in the DTC television advertisement.
To communicate this information in the DTC television advertisement in a manner that will facilitate consumer comprehension, the firm presents the absolute risk percentages in direct conjunction with the 50% relative risk reduction information and with equal prominence: In a clinical trial, Drug X reduced the risk of stroke by 50% (1% of patients treated with Drug X had a stroke, compared to 2% of patients in the control group).
C. Formatting Quantitative Efficacy or Risk Information
Firms that provide quantitative efficacy or risk information about their drugs in DTC promotional communications should incorporate the following formatting recommendations:
Present the information in the same numerical format throughout a promotional communication (Lipkus 2007; Trevena et al. 2013). For example, firms providing two probabilities about two efficacy outcomes should provide both probabilities as absolute frequencies or provide both probabilities as percentages. Firms should also consistently characterize efficacy or risk information quantitatively throughout a promotional piece, rather than alternating between qualitative descriptors and quantitative information to describe similar information or concepts.
Use frequencies with the same denominator when providing more than one absolute frequency and consider using denominators that are multiples of 10 (Fagerlin et al. 2007; Lipkus 2007; Trevena, et al. 2013; Visschers et al. 2009).
Express probabilities using whole numbers to the extent that the probabilities in whole numbers accurately reflect the numerical value being described in the promotional piece (Lipkus 2007; Zipkin et al. 2014).9 Where a whole number would not be appropriate, firms should express the value as is (e.g., as a decimal) instead of rounding the value up or down to the nearest whole number. For example, firms should not round probabilities less than 1 to the nearest whole number. Similarly, firms should not round probabilities to the nearest whole number when comparing probabilities that are so close in value that the difference between the probabilities would be lost if the values were expressed as a whole number or numbers.
Promotional communications should present quantitative probability information about a particular risk in a manner that does not minimize or detract from information about the severity of the risk. Promotional communications should avoid presentations that focus attention on the low probability of a serious risk occurring, that characterize the probability of that risk occurring as insignificant, or that otherwise suggest that the risk is not important based on its probability of occurring.
Example 5: A firm is developing a consumer brochure for Drug X and is considering whether to describe quantitative information about moderate symptom relief in patients treated with Drug X and treated with placebo in terms of absolute frequencies (9 out of 10 and 3 out of 10, respectively) or as percentages (90% and 30%, respectively).
Although either probability measure would be appropriate to describe these outcomes, to help consumers process the information, the firm should provide the outcomes for both the treatment and placebo groups in the same format (i.e., both outcomes as absolute frequencies or both outcomes as percentages): In patients treated with Drug X, 9 out of 10 patients experienced moderate symptom relief, compared to 3 out of 10 patients who received placebo. Alternatively: In patients treated with Drug X, 90% of patients experienced moderate symptom relief, compared to 30% of patients who received placebo.
Example 6: In a clinical trial for Drug X, 54% of patients treated with Drug X experienced moderate symptom relief and 19% of patients treated with Drug X experienced complete symptom relief, compared to 28% of patients treated with placebo and 2% of patients treated with placebo, respectively.
The firm is developing a patient booklet for Drug X that contains the following information: In a clinical trial, the majority of patients experienced moderate symptom relief after treatment with Drug X, and 19% of patients experienced complete symptom relief. In patients treated with placebo, less than half of patients experienced moderate symptom relief and 2% of patients experienced complete symptom relief.
To present the information consistently, the firm should include the “majority of patients (54%)” and “less than half of patients (28%)” in the proposed patient booklet. Alternatively, the firm could consistently present only the quantitative information throughout the piece (e.g., “…54% of patients treated with Drug X experienced moderate symptom relief...,” “...28% of patients treated with placebo experienced moderate symptom relief...”).
Example 7: According to the FDA-approved labeling for Drug X, 2% of clinical trial
participants on Drug X experienced bleeding that required hospitalization.
In its promotional communications for Drug X, the firm includes the statement, “In a clinical trial, only 2% of patients experienced bleeding that required hospitalization.”
By including the qualifier only in the description of the percentage of patients who experienced bleeding that required hospitalization, the presentation characterizes the percentage of patients who experienced this risk in a way that could suggest it is not important and may also undermine audience understanding of the serious nature of the risk. To avoid these possibilities, the firm should revise this presentation to remove the qualifier “only.”
D. Visual Aids
When DTC promotional communications contain quantitative efficacy or risk information, visual aids such as graphs, tables, and icon arrays are often used to illustrate the information and put the numerical values in context. Visual representations of efficacy and risk in DTC promotional communications improve consumer comprehension of numeric values by illustrating patterns, summarizing the data, and reducing the amount of mental calculations the consumer must perform to extract meaning from the quantitative information (Ancker et al. 2006; Fagerlin et al. 2007; Lipkus 2007). Moreover, visual aids can improve consumers’ ability to accurately understand how well a drug works and support decision making (Fagerlin et al. 2007; Garcia-Retamero and Cokely 2013; Sullivan et al. 2016; Zipkin et al. 2014).
Visual aids in DTC promotional communications help consumers comprehend quantitative efficacy and risk information, but all visual aid designs are not equally effective in conveying all types of information (Fagerlin et al. 2007; Sullivan et al. 2016). Therefore, we recommend that firms select the visual aid design that best communicates the quantitative efficacy or risk information being presented. When choosing a visual aid to express quantitative efficacy or risk information about a drug, firms should carefully consider the communication’s purpose and objectives (Ancker et al. 2006; Fagerlin et al. 2007). For example, a bar graph is an appropriate format for visually depicting comparisons between probabilities, whereas a line graph is more useful for illustrating trends or changes over time (Ancker et al. 2006; Fagerlin et al. 2007; Lipkus 2007). Additionally, firms should consider the following general recommendations when designing visual aids to illustrate quantitative efficacy or risk information in their DTC promotional communications:
Explain the purpose of the visual aid clearly and accurately, and define the elements displayed (Garcia-Retamero and Cokely 2013; Lipkus 2007). For example, firms should include a title, header, or caption (written or oral depending on the media) and identify the visual aid’s variables, scales, and axes (when applicable).
Make visual displays of numeric information proportionate to the quantity being described and ensure the scaling of axes is appropriate to accurately represent effect sizes (Ancker et al. 2006; Lipkus 2007). For example, the height of each bar on a vertical bar graph should be proportionate to the numerical value it represents and the scaling of the y axis should ensure the difference in heights between bars is proportional to the difference in value.
Include visual representations of both the numerator and denominator of ratios or frequencies (Ancker et al. 2006). For example, an icon array, graph, or other visual aid depicting an absolute frequency should represent the people who experienced the effect (numerator) and the total people studied (denominator). When possible, firms should also consider illustrating the denominator as a multiple of 10 in the display.
Example 8: Drug X is used to treat a serious condition. Infection is a risk associated with the use of Drug X.
The firm intends to include a visual aid on Drug X’s consumer website to communicate information from Drug X’s FDA-approved labeling about the percentage of patients who experienced a mild to moderate infection and those who experienced a severe to life-threatening infection after treatment with Drug X compared to patients treated with placebo.
The firm prepares a bar graph to present this information because it facilitates the comprehension of visual comparisons between probabilities. As illustrated below, the firm includes a title that describes what the bar graph portrays, labels the scales and variables, does not truncate the y axis, and ensures that the values graphically displayed are proportionate to the quantities being described.
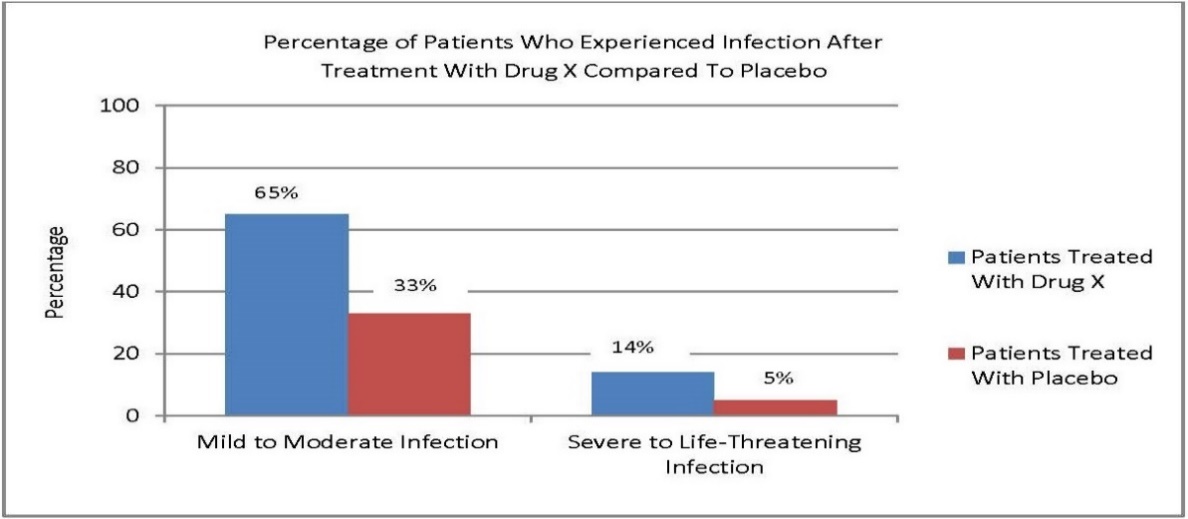
IV. PaperWORk REDUCTIon Act of 1995
This guidance contains information collection provisions that are subject to review by the Office of Management and Budget (OMB) under the Paperwork Reduction Act of 1995 (44 U.S.C. 3501-3521).
The time required to complete this information
collection is estimated to average 2 hours per response, including
the time to review instructions, search existing data sources, gather
the data needed, and complete and review the information collection.
Send comments regarding this burden estimate or suggestions for
reducing this burden to:
Office of Prescription Drug Promotion, Center for Drug Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Avenue, Bldg. 51, Silver Spring, MD 20993-0002.
This guidance also refers to previously approved FDA collections of information. The collections of information in 21 CFR 202.1 have been approved under OMB control number 0910-0686.
An Agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The OMB control number for this information collection is 0910-0686 (expires 08/31/2024).
References
Ancker, JS, Y Senathirajah, R Kukafka, and JB Starren, 2006, Design Features of Graphs in Health Risk Communication: A Systematic Review, J Am Med Inform Assoc, 13(6):608–618.
Buchter, RB, D Fechtelpeter, M Knelangen, M Ehrlich, and A Waltering, 2014, Words or numbers? Communicating Risk of Adverse Effects in Written Consumer Health Information: A Systematic Review and Meta-Analysis, BMC Med Inform Decis Mak, 14:76.
Covey J, 2007, A Meta-Analysis of the Effects of Presenting Treatment Benefits in Different Formats, Med Decis Making, 27(5):638–654.
Fagerlin, A, PA Ubel, DM Smith, and BJ Zikmund-Fisher, 2007, Making Numbers Matter: Present and Future Research in Risk Communication, Am J Health Behav, 31(Suppl 1):S47–56.
Garcia-Retamero, R and ET Cokely, 2013, Communicating Health Risks with Visual Aids, Curr Dir Psychol Sci, 22(5):392–399.
Lipkus, IM, 2007, Numeric, Verbal, and Visual Formats of Conveying Health Risks: Suggested Best Practices and Future Recommendations, Med Decis Making, 27(5):696–713.
O’Donoghue, AC, HW Sullivan, and KJ Aikin, 2014a, Randomized Study of Placebo and Framing Information in Direct-to-Consumer Print Advertisements for Prescription Drugs, Ann Behav Med, 48(3):311–322.
O'Donoghue, AC, HW Sullivan, KJ Aikin, D Chowdhury, RR Moultrie, and DJ Rupert, 2014b, Presenting Efficacy Information in Direct-to-Consumer Prescription Drug Advertisements, Patient Educ Couns, 95(2):271–280.
Schwartz, LM, S Woloshin, and HG Welch, 2007, The Drug Facts Box: Providing Consumers with Simple Tabular Data on Drug Benefit and Harm, Med Decis Making, 27:655–662.
Schwartz, LM, S Woloshin, and HG Welch, 2009, Using a Drug Facts Box to Communicate Drug Benefits and Harms: Two Randomized Trials, Ann Intern Med, 150(8):516–527.
Sullivan, HW, AC O’Donoghue, and KJ Aikin, 2013, Presenting Quantitative Information About Placebo Rates to Patients, JAMA Intern Med, 173(21):2006–2007.
Sullivan, HW, AC O’Donoghue, and KJ Aikin, 2015, Communicating Benefit and Risk Information in Direct-to-Consumer Print Advertisements: A Randomized Study, Ther Innov Regul Sci, 49(4):493–502.
Sullivan, HW, AC O’Donoghue, KJ Aikin, D Chowdhury, RR Moultrie, and DJ Rupert, 2016, Visual Presentations of Efficacy Data in Direct-to-Consumer Prescription Drug Print and Television Advertisements: A Randomized Study, Patient Educ Couns, 99:790–799.
Sullivan, HW, AC O’Donoghue, M Lynch, Mihela Johnson, C Davis, and DJ Rupert, 2019, The Effect of Including Quantitative Information on Multiple Endpoints in Direct-to-Consumer Prescription Drug Television Advertisements, Med Decis Making, 39(8):975–985.
Trevena, LJ, BJ Zikmund-Fisher, A Edwards, W Gaissmaier, M Galesic, PKJ Han, J King, ML Lawson, SK Linder, I Lipkus, E Ozanne, E Peters, D Timmermans, and S Woloshin, 2013, Presenting Quantitative Information About Decision Outcomes: A Risk Communication Primer for Patient Decision Aid Developers, BMC Med Inform Decis Mak, 13(Supple 2):S7.
Visschers, VH, RM Meertens, WW Passchier, NN De Vries, 2009, Probability Information in Risk Communication: A Review of the Research Literature, Risk Anal, 29(2):267–287.
West, SL, LB Squiers, L McCormack, BG Southwell, ES Brouwer, M Ashok, L Lux, V Boudewyns, A O'Donoghue, and HW Sullivan, 2013, Communicating Quantitative Risks and Benefits in Promotional Prescription Drug Labeling or Print Advertising, Pharmacoepidemiol Drug Saf, 22(5):447–458.
Woloshin, S, LM Schwartz, and HG Welch, 2004, The Value of Benefit Data in Direct-to-Consumer Drug Ads, Health Aff, Suppl Web Exclusives, W4:234–245.
Zipkin, DA, CA Umscheid, NL Keating, E Allen, K Aung, R Beyth, S Kaatz, DM Mann, JB Sussman, D Korenstein, C Schardt, A Nagi, R Sloane, and DA Feldstein, 2014, Evidence-Based Risk Communication: A Systematic Review, Ann Intern Med, 161(4):270–280.
1 This guidance has been prepared by the Office of Prescription Drug Promotion in the Center for Drug Evaluation and Research in consultation with the Center for Biologics Evaluation and Research and the Center for Veterinary Medicine at the Food and Drug Administration.
2 While this guidance focuses on quantitative presentations of efficacy and risk information, firms may wish to refer to the principles and recommendations for quantitative presentations of other product benefits (keeping in mind that any such presentation of other product benefits otherwise must comply with applicable statutory and regulatory requirements).
3 The term drugs in this guidance refers to prescription human drug and biological products and to prescription and over-the-counter animal drugs.
4 Promotional labeling is generally any labeling other than the FDA-required labeling. Promotional labeling can include printed, audio, or visual matter descriptive of a drug that is disseminated by or on behalf of that drug’s manufacturer, packer, or distributor (21 CFR 202.1(l)(2)). The Federal Food, Drug, and Cosmetic Act (FD&C Act) does not define what constitutes an advertisement for a prescription drug, but FDA regulations provide several examples (21 CFR 202.1(l)(1)).
5 The term firms in this guidance refers to manufacturers, packers, and distributors of prescription drugs (as described in this guidance) and over-the-counter animal drugs, including their representatives.
6 This guidance is not intended to describe whether or when a presentation of quantitative efficacy or risk information would be truthful or non-misleading. FDA reminds firms that they are responsible for ensuring that their promotional materials are truthful and non-misleading and that they comply with applicable statutory and regulatory requirements. See, for example, sections 201(n) and 502(a) and (n) of the FD&C Act (21 U.S.C. 321(n), 352(a) and (n)); 21 CFR 1.21(a) and 202.1(e)(5). Additionally, we note that there may be ways other than the recommendations provided in this guidance that would make presentations of quantitative efficacy or risk information consumer-friendly.
7 See sections 201(n) and 502(a) and (n) of the FD&C Act (21 U.S.C. 321(n), 352(a) and (n)); 21 CFR 1.21(a) and 202.1(e)(5).
8 Each of the examples in this guidance is intended to stand on its own, and the use of ‘Drug X’ represents a different fictitious drug in each example.
9 For values greater than 1, to express a value to the nearest whole number, the following principles should be followed: For amounts falling exactly halfway between two whole numbers or higher (e.g., 2.5 to 2.99), round up (i.e., 3); for values less than halfway between two whole numbers (e.g., 2.01 to 2.49), round down (i.e., 2).

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Guidance for Industry |
| Author | Rogers, John * |
| File Modified | 0000-00-00 |
| File Created | 2024-09-20 |
© 2026 OMB.report | Privacy Policy