Attachment F. Moderator Guide
FDA Focus Groups and Interviews
Attachment F. Moderator Guide
OMB: 0910-0497
Attachment F
OMB Control No. 0910-0497
Expiration Date: 12/31/2026
Paperwork Reduction Act Statement: According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a valid OMB control number. The time required to complete this information collection is estimated to average 60 minutes per response, including the time for reviewing instructions and completing and reviewing the collection of information.
Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden, to PRAStaff@fda.hhs.gov.
This study is being conducted on behalf of the U.S. Food and Drug Administration.
Focus Groups Examining Consumer Reporting of Adverse Events
Moderator Guide
A. WELCOME AND GROUND RULES (5 min)
Thank you for taking the time to join us today. My name is [NAME] and I work for a company called Westat, we are based in Rockville, Maryland. We do a lot of research for the federal government and today we are conducting a research study on behalf of the U.S. Food and Drug Administration – or the FDA. The purpose of today’s discussion is to learn about negative side-effects – what you might hear me refer to as an “adverse event” – you may have had from human drug products. By “human drug products,” I mean a prescription drug or drug that you were able to buy at a pharmacy without a prescription, or what are called “over-the-counter” or “non-prescription” drugs. I’ll also be interested in learning about who, if anyone, you talked to about that bad experience. We’re also going to look at some sections of the website for reporting these kinds of events and ask for your feedback. FDA is interested in finding ways to make the reporting of these drug-related events easier for consumers, so your feedback is very important to us. Our discussion will last about 60 minutes. Before we get started there are a few things I need to mention.
First, your participation is voluntary. You don’t have to answer any of our questions that make you uncomfortable, and you can leave at any point in the discussion. There’s no penalty to you for doing so.
Having said that, we think there are minimal risks to you for participating in today’s discussion. We’re not going to ask you for any personal health information, like what specific drugs you are taking for what health conditions. We’re going to ask you to talk generally about any negative experiences you may have had with human drug products. So, for example, I might say, “My doctor prescribed me something and the first day I took it, I woke up on the bathroom floor. I don’t even remember falling, but I have to believe it was that medication she put me on that did it.” So, again, just very general information.
There are no particular benefits to you, either, for participating, although you will be helping FDA make it easier for consumers to report any negative experiences, they may have had with drugs or medications.
Next, everything you say here today will be kept secure to the extent provided by law, which means we will never use your name in anything we write about the project. When preparing our report for FDA, we might use a quote from someone in the group, but we would only say, “A participant in a group on the East coast said --.”
We have some virtual observers today from the project team at FDA. All staff who are observing must keep the identity of respondents secure to the extent permitted by law. They may have additional questions for you, so I’ll check in with them at the end of the group for those. You can also see my colleague, [NAME], on your screen. She is there to assist you if something goes wrong with your technology; she can also take over for me if something goes wrong with my technology.
We would also like to audio- and video-record today’s discussion. This is so we have an accurate record of what was said when we write our report for FDA. The files are stored on secure servers at Westat and FDA, are only available to members of the project team, and will be destroyed once the project is completed. Before I start the recording, though, I would like to see if anyone has any questions? [ANSWER ANY QUESTIONS] Do I have everyone’s permission to record? [MAKE SURE YOU GET NODS OR “OKAYS” BEFORE PRESSING The RECORD BUTTON]
Let me tell you just a couple of rules to make sure our discussion flows smoothly today. If you could, please speak one at a time. This will ensure that I can hear everybody and that everyone’s perspective can be included in our study. Also, there are no right or wrong answers to any of the questions I ask. Feel free to express an opinion that’s different from others on the line – we want to hear the full range of views on this topic. In addition, I am not an expert on what we’re going to be talking about today; you may have questions as we go along that I can’t answer, but please ask them so FDA can hear what questions you have. As we discussed during your tech check, we know it’s difficult to find private space when joining from home. But please do your best to keep others in your household from seeing your screen or hearing the conversation. And if you need to leave the discussion for a moment or have a lot of background noise, please mute yourself (but stay unmuted otherwise). Finally, if you need to step out for any reason, please feel free to do so and come back as soon as you can.
Any questions? [ANSWER ALL QUESTIONS]
Let’s get started!
B. PARTICIPANT INTRODUCTIONS – WARM-UP (5 min)
[MODERATOR – DECIDE WHO WILL GO FIRST IN The GROUP AND GIVE THAT PERSON A HEAD’S-UP] Tell us your first name only, the state you’re calling in from, and something unique or interesting about yourself – a hobby, the coolest place you’ve visited, somebody famous you may have run into. [MODERATOR – GO LAST]
C. PARTICIPANT EXPERIENCES WITH MEDICATION-RELATED ADVERSE EVENTS (20 min)
As I mentioned at the beginning of the group, we’re going to focus today on your experiences with different human drug products. This includes drugs that you can buy at the store yourself, like Tylenol, cold and flu medications, etc. And it includes those drugs or medications for which your doctor wrote up a prescription and a pharmacist had to fill.
Thinking about conversations you’ve had with family members or friends, or maybe things you’ve seen on social media, what kind of unwanted experiences or bad side effects have you heard about people having from taking a human drug product?
[IF PERSONAL EXPERIENCES HAVE NOT ALREADY COME UP] Now, thinking about your own experiences with human drug products, has anyone here had an unwanted experience or side effect? Again, you don’t need to name the drug specifically or the condition for which you were taking it, but if you could, please tell us about that experience.
What did you do after you had that experience? For example, did you tell anyone about it or report the experience to anyone?
TELL SOMEONE: IF YES: Tell us about that [Probe on who the person talked to; what happened after talking about the incident; what happened to the information after telling someone? How long after the incident did you tell someone?]
REPORT IT: IF YES: Tell us about that [Probe on who they reported the event to; what happened after reporting the incident, what do you think happened to that information after reporting it? How long after the incident did you report it.]
IF NO: DID NOT TELL SOMEONE OR REPORT: Can you say a little bit about why you did not tell anyone or report it?
For those of you who have not had an unwanted reaction or bad side effects from a human drug product, do you think you would:
TELL SOMEONE:
If yes, who would you tell?
If no, why wouldn’t you tell anyone?
REPORT IT:
If yes, why would you report? To whom (could be an individual or an organization) would you report? When would you report (e.g., as soon as it happens? Or later)?
If no, what would prevent you from reporting ?
Are there any circumstances under which you might tell someone about or report an event? [IF NEEDED: For example, what kinds of side effects would you let someone know about? [IF NEEDED: How serious does the side effect need to be?] [IF NEEDED: For example, you reported it to someone, but you feel they didn’t do anything about it.] Who would you report it to?
What kinds of side effects might you not tell anyone about? Why would you not report those? [IF NEEDED: Would you report a side effect that the product label warns the user about? Why/not?]
IF NOT MENTIONED: Would anyone report the event to the FDA? Why or why not?
Are you familiar with/ever heard of a program called MedWatch? IF YES, What do you know/have you heard about it? IF NO, What do you think MedWatch is?
D. MedWatch Explanation (2 min)
Today we’re going to talk about a program called, “MedWatch.” MedWatch is the FDA program for healthcare providers, patients, and consumers to report adverse events and product quality problems associated with FDA-regulated products for human use. Examples of products that can be reported through MedWatch include prescription and over-the-counter medicines, biologics, medical devices, combination products, special nutritional products, infant formulas, cosmetics, and foods. FDA depends on the voluntary reporting of adverse events and problems with medical products by patients and consumers to keep effective medical products available on the market.
[MODERATOR: Display slide with the names of the different products that can be reported. Ask participants how they understand each of the terms.]
DRAFT SLIDE
Prescription
medicine Over-the-counter
medicine Biologics Medical
devices Combination
products Special
nutritional products
Infant
formulas Cosmetics Foods
E. MedWatch Form (20-25 min)
[MODERATOR – Use screen sharing feature on Zoom to show participants the introductory page for MedWatch: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm. You will need to enlarge the page to at least 125% for it to be readable for most participants.]
1. Introductory Page and Contact Information

You all have talked about different circumstances under which you might file a report about an adverse event [MODERATOR – repeat a couple of those circumstances back to participants.] So, let’s say one of those things happened to you and you want to file a report with FDA. You would start off on this page. There is one thing I would like to look at on this page, which is this section that reads [USE POINTER TO INDICATE The SENTENCES], “While not mandatory, FDA encourages….”
I’m interested in hearing your thoughts about or any reactions you might have to these two sentences. [PROBE IF NEEDED: How comfortable are you with submitting your contact information to FDA? Explain. How would you feel about FDA submitting your contact information to the manufacturer? Explain. For those of you who are not comfortable, what are your thoughts about seeing that you can request FDA not share this information?]
2. What kind of problem was it?

Take a minute to look at these response options. Are there other types of problems that you think should be added to the list? IF YES, Explain.
I’d like to look specifically at the second option, “Used a product incorrectly….” Would you answer this question if you had used a product incorrectly? Explain. Would you continue filling out the form? Why/not?
3. Outcomes

Let’s take a look at this list of things that might happen as a result of an adverse drug event. First, I’m interested in hearing your reactions to this list. [IF NEEDED: Is there anything on the list that surprises you?] Are there other things that you think could happen that you believe should be on this list? [Describe/explain] If that were to happen to you or a family member, what would you do when you came to this question on the reporting form?
4. Relevant Laboratory Tests
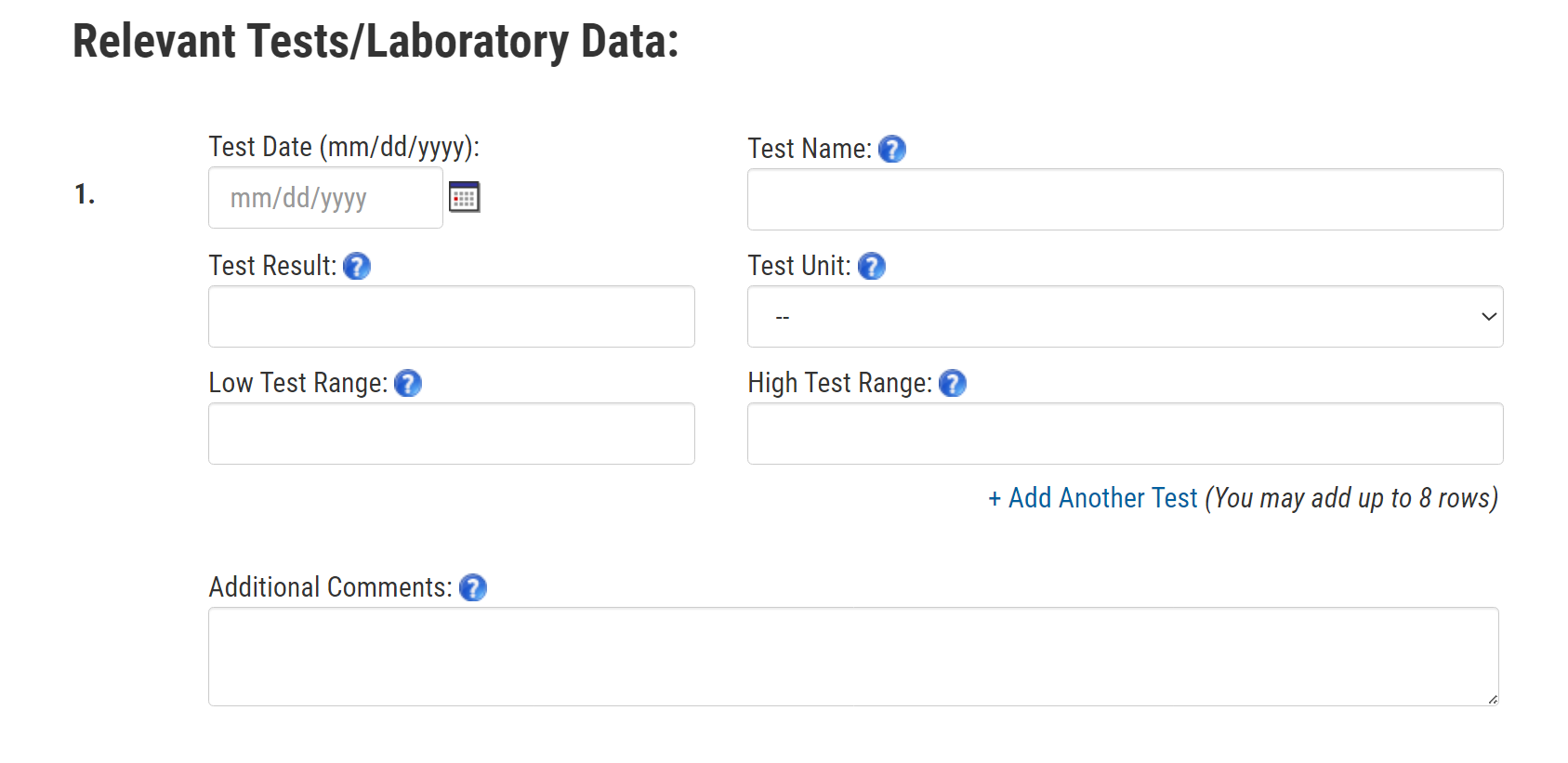
Let’s take a look at this section on laboratory test data. What would you do when you came to this section of the reporting form? [PROBE, IF NEEDED: Would you try to find or obtain the lab data? If so, how might you do that?] What would you do if you didn’t have this information? [PROBE: Skip this section and continue? Quit filling out the form?]
5. Cause of the Problem and Product Information
MODERATOR: Show participants the section (below) of the online form, select the first option (and explain why), then move to the next page.

What would you select when you got to this section (below) of the form? Explain. [PROBE IF NEEDED: What would you do if you were not certain which box to check? Continue without checking anything? Quit filling out the form?]

6. Product Type

Let’s look at these categories of product types. I’m interested in hearing what, if anything, each of these means to you. [MODERATOR: Start with OTC – ask participants how they might explain that category to someone else. Continue.]
Let’s say you are given a prescription for a name-brand drug by your doctor [MOD – reference discussion about generics for contrast]. You take that prescription to your local CVS or Walgreen’s to be filled, and that drug ends up causing you an unwanted side effect – an adverse event – that you want to report. What would you select on this form? Why? IF NEEDED: What would you do if you were uncertain about what to check? [Continue without answering? Quit filling out the form?]
7. About Patient
[MODERATOR: Scroll down the page to show participants all of the information that is being requested.]

I’m interested in any thoughts or reactions you might have to this page. What would you do if you were completing this form for yourself? [PROBE: Fill it out? Skip it? Quit filling out the form altogether?] What about if you were filling it out for someone else? [PROBE: Fill it out? Skip it? Quit filling out the form altogether?]
8. Ethnicity and Race
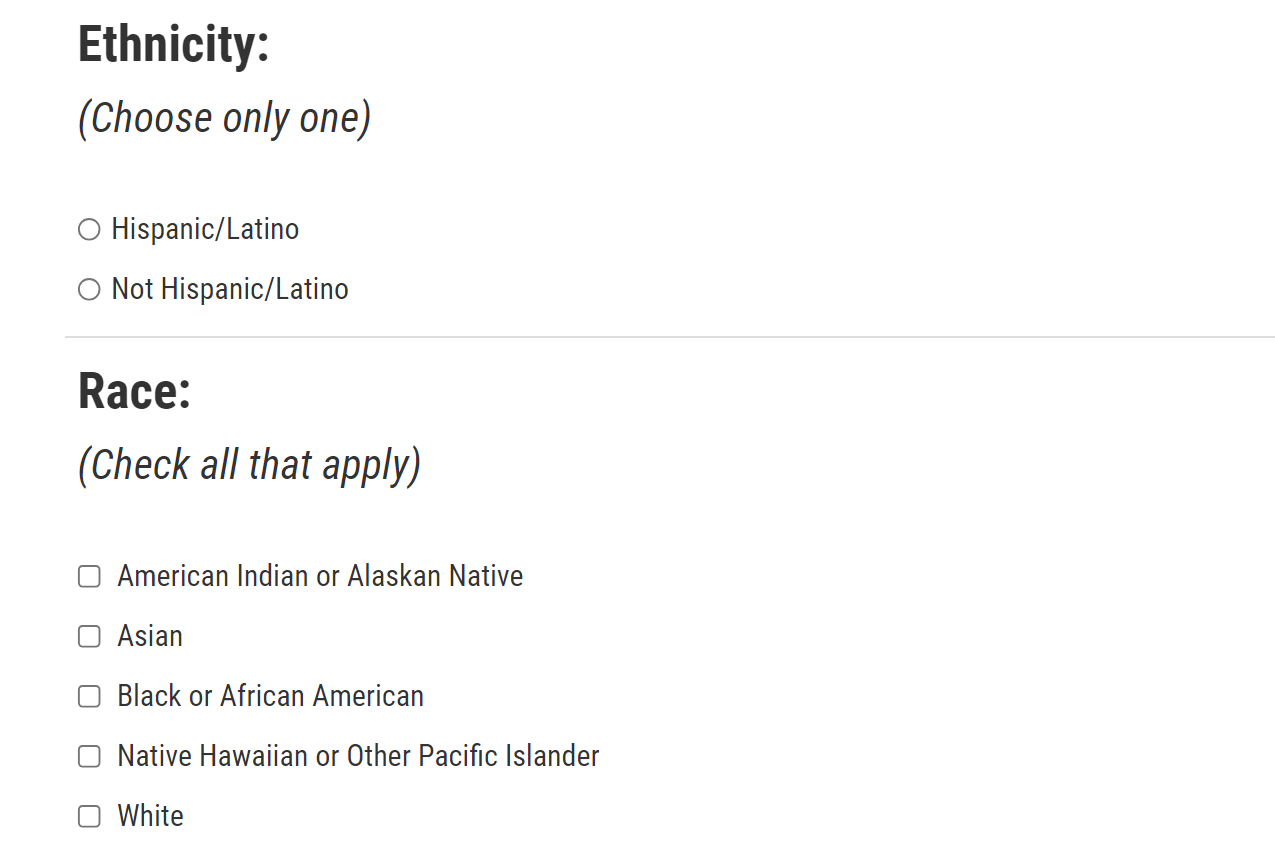
This section is still asking for information about the patient. If you are the patient and you’re completing your own report, how well do the categories here reflect how you think about yourself? How comfortable would you feel sharing this information? Explain. If you were not comfortable sharing this information, what would you do next? [PROBE: Skip the section or quit filling out the form? Explain.]
9. About Reporter
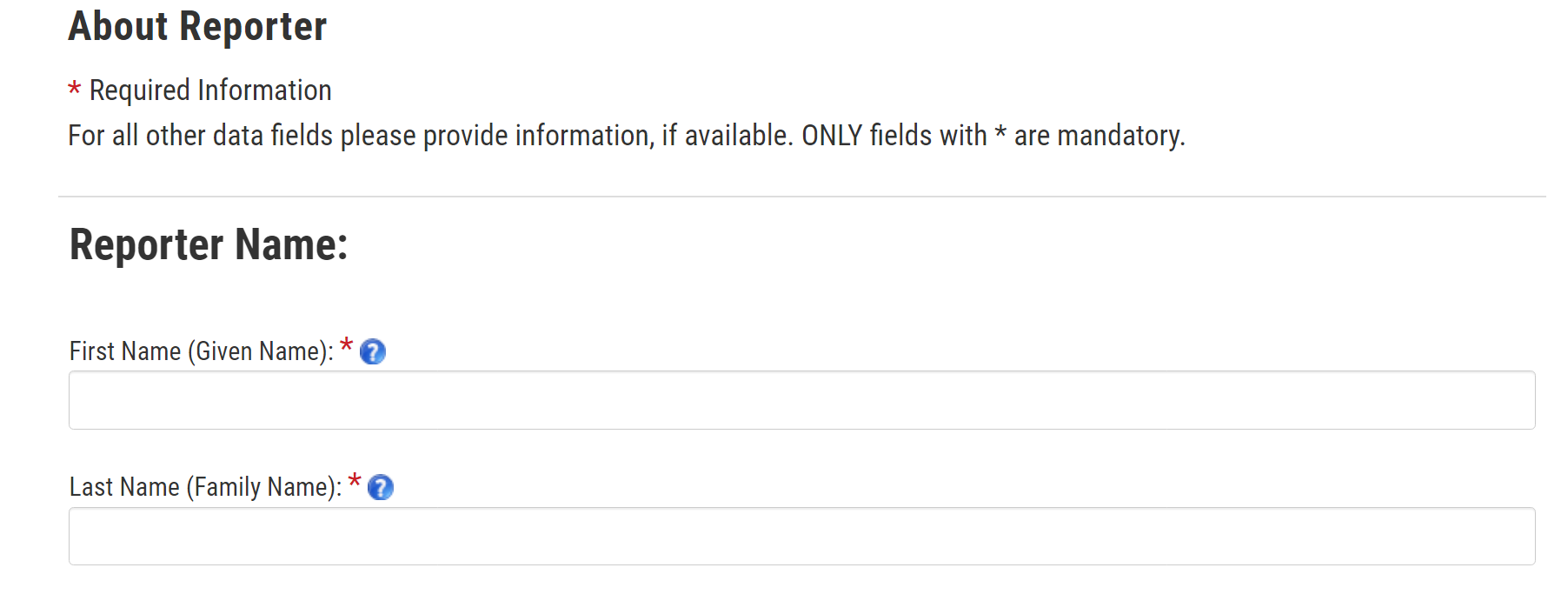
What would you do if you were completing this form for yourself? [PROBE: Skip it? [can’t skip, so…?] Fill in someone else’s information? Drop off from completing the report?] What would you do if you were filling this form out for a family member?
10. Identity Disclosure

This box comes right before you get the opportunity to review your data and submit the report to MedWatch. Would you check the box or not? Explain.
We’ve reviewed several sections of the MedWatch reporting form and you have offered some very important insights into how easy or difficult it could be to fill out certain sections and [IF APPROPRIATE TO The GROUP] where some of you might decide to stop filling out the form altogether. Now that you’ve seen much of what would be required to file a report about an adverse drug event, is there anything else that might prevent you from filing a report that we haven’t yet talked about?
F. Close (2-3 min)
Those are all the questions I have for you. While I wait to see if my colleagues at FDA have any follow-up questions they would like me to ask you, I am going to put into the chat box the link to the MedWatch form that we just reviewed (https://www.accessdata.fda.gov/scripts/medwatch/index.cfm). Then, if you or someone you know has an adverse event in the future and you would like to report it, you’ll know where you can go to let FDA know about the incident.
[MODERATOR – Ask any follow-up questions from observers.]
That ends our discussion today. Thank you so much for your time and your valuable feedback! PRC will be sending you the incentive check – it usually takes about 2 weeks. Have a great evening!
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Cynthia Robins |
| File Modified | 0000-00-00 |
| File Created | 2024-09-11 |
© 2026 OMB.report | Privacy Policy