eHCTERS Screenshots_07 11 2023
Human Cells, Tissues, and Cellular and Tissue-Based Products
eHCTERS Screenshots_07 11 2023
OMB: 0910-0543
CBER On-Line- Login Screen

CBER On-Line Main Menu Screen
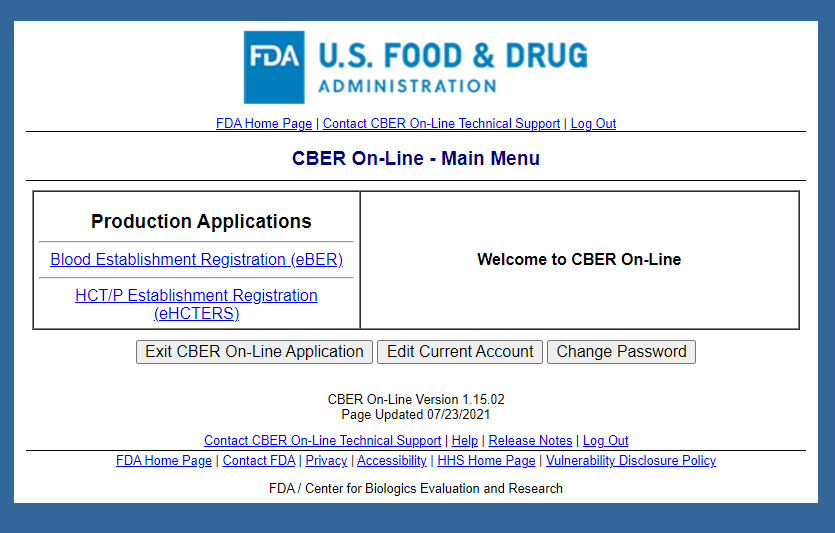
Note: After eHCTERS application is selected, the OMB control number and expiration date are displayed on all subsequent pages (see next page for an example).
Electronic Human Cells and Tissue Establishment Registration System (eHCTERS)
Activity Selection Screen (screenshot of the entire page)
UserName



The text highlighted in yellow will be replaced with:
OMB Control Number 0910-0543; Expiration Date 07/31/2023
See OMB Burden Statement
The hyperlink will lead to this text:
OMB Burden Statement
Public reporting burden for this collection of information is estimated as follows:
The time required to complete registration and listing for existing establishments is estimated to average 30 minutes per response, for new establishments it is estimated to average 45 minutes per response, and updates to a list are estimated to average 30 minutes per response, including the time to review instructions, search existing data sources, gather and maintain the data needed and complete and review the collection of information.
Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden, to PRAStaff@fda.hhs.gov.
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number.
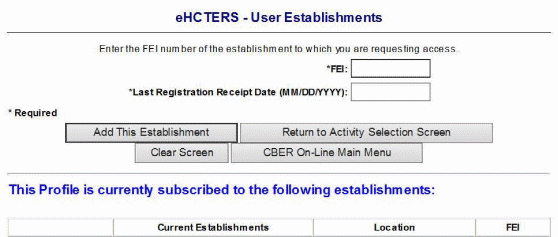
User Establishments Screen
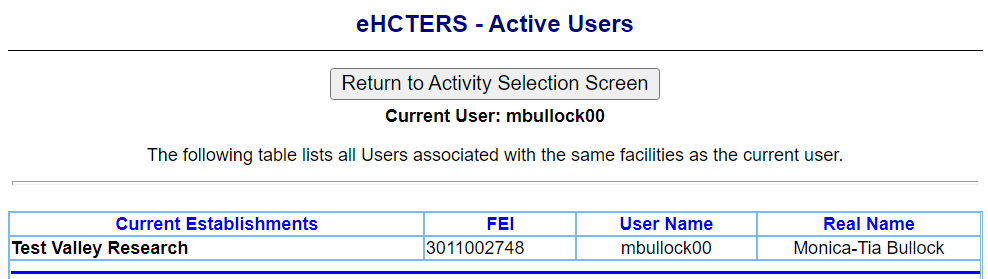
Active Users Screen






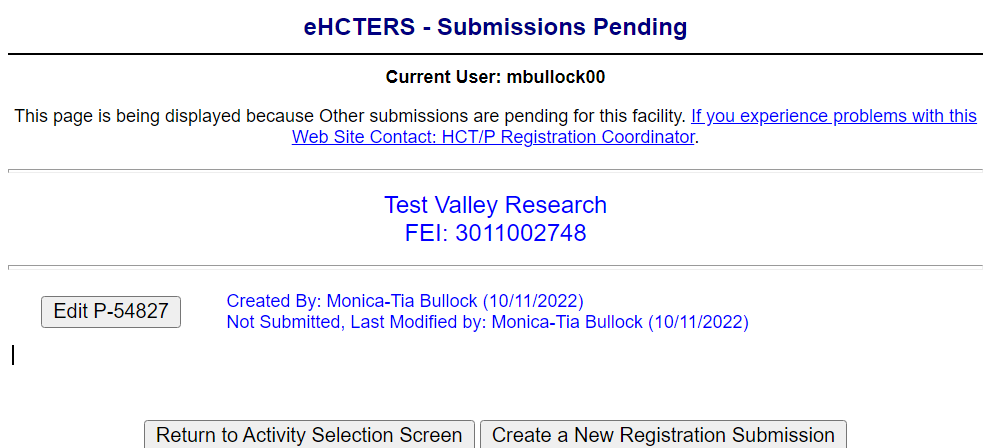
S

 ubmissions
Pending Screen
ubmissions
Pending Screen








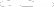












Initial Establishment Registration Information Screen
Edit Establishment Registration Information Screen

Establishment Registration Address Information Screen

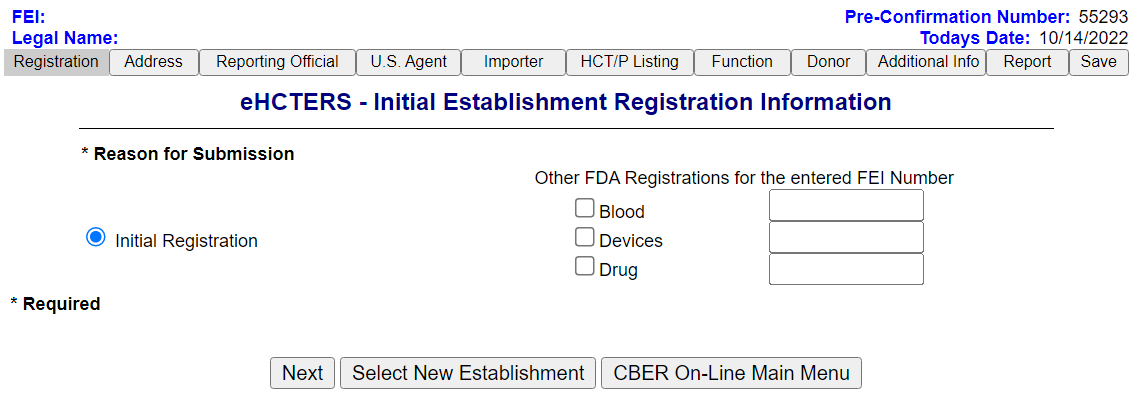
Possible Establishment Matches Found Screen
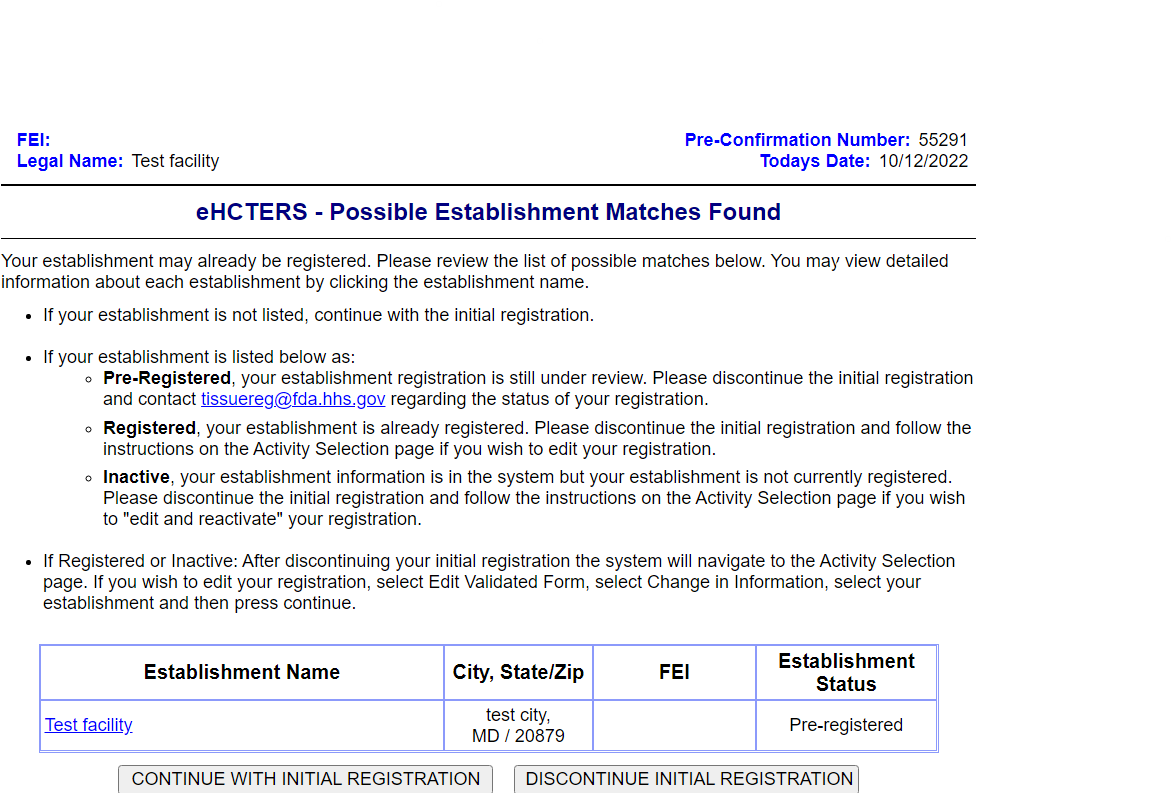
Establishment Reporting Official Information Screen
Establishment U.S. Agent Information Screen

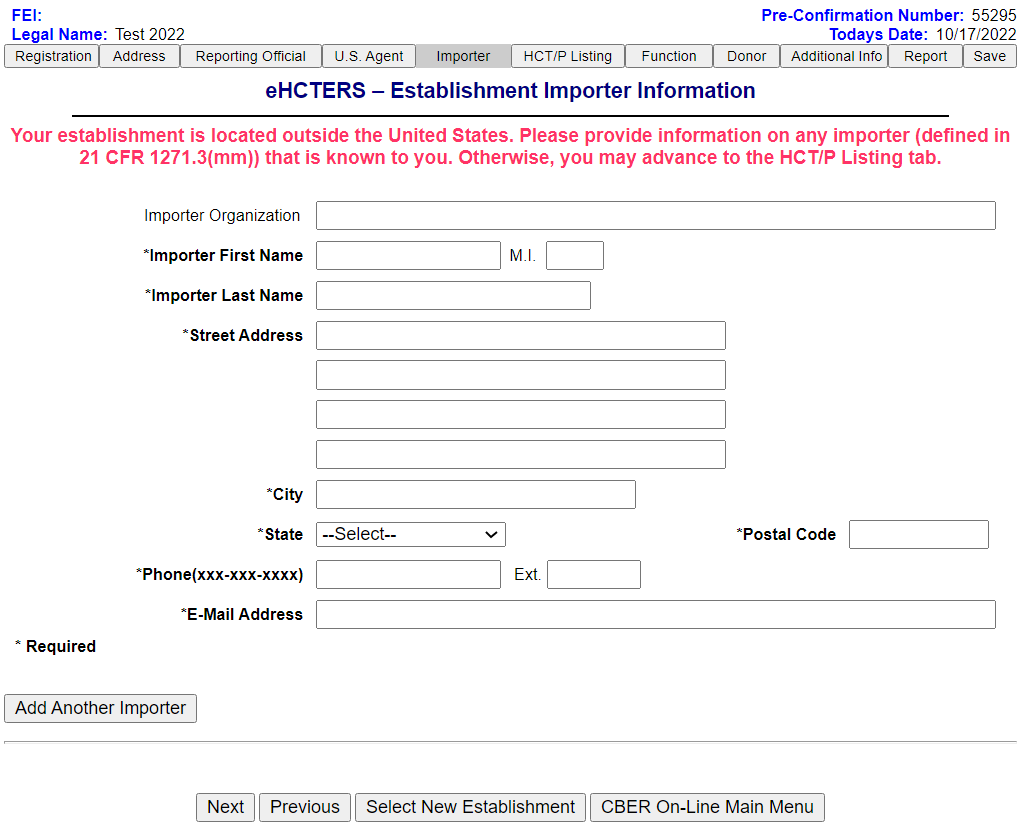
Establishment Importer Information Screen
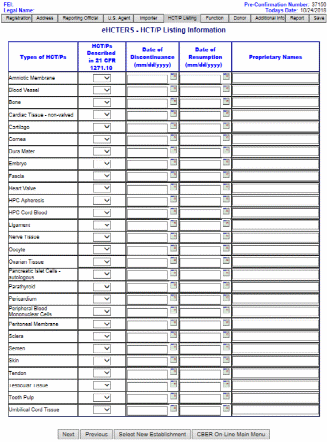
HCT/P Listing Information Screen
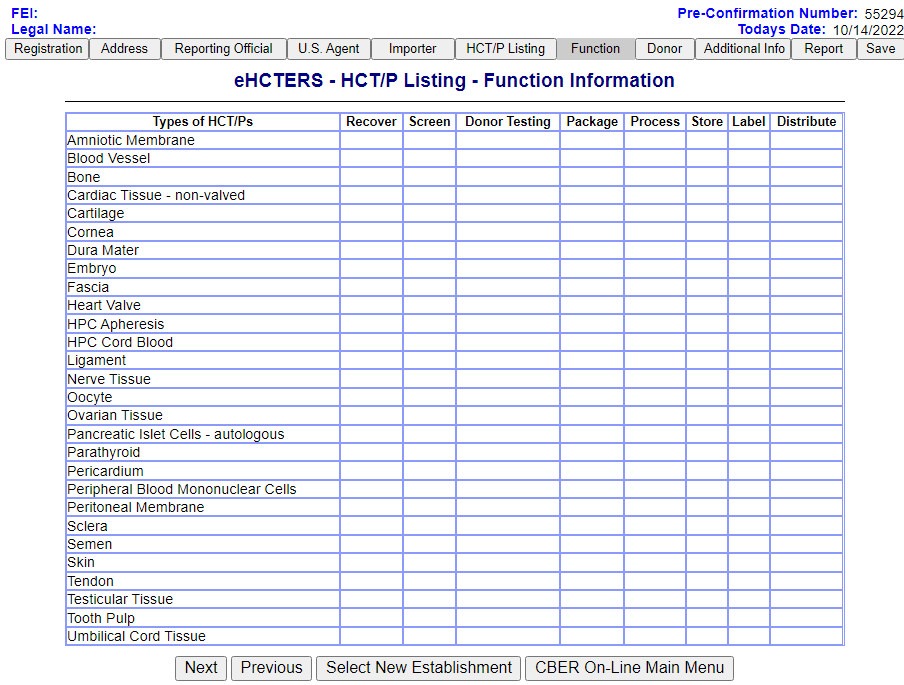
Function Information Screen
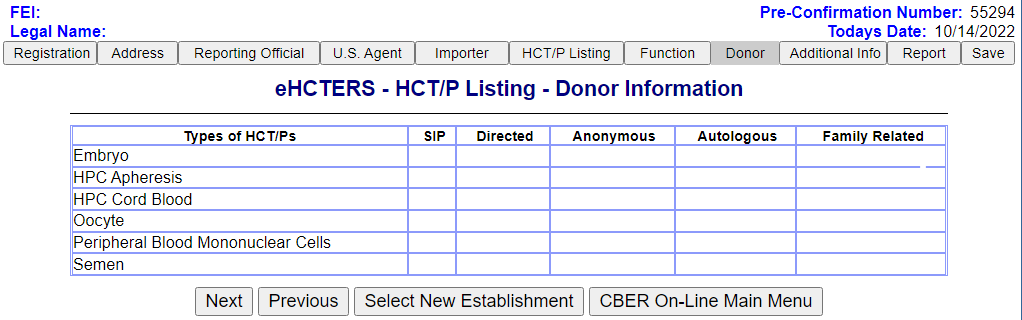
Donor Information Screen
Additional Information to Complete HCT/P Information Listing Screen

Reporting Official Signature Screen
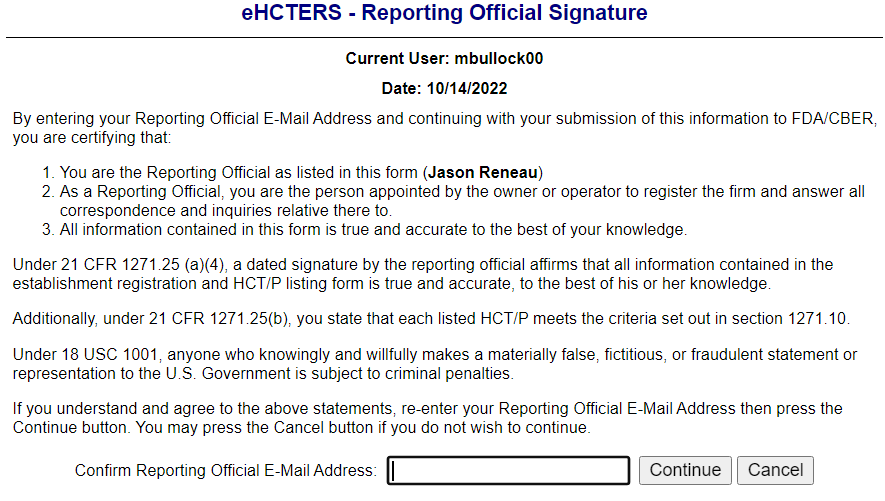



Submitted Establishment Registration Information Screen
Registration Summary Report Provided to the Establishments (Example-Page 1)
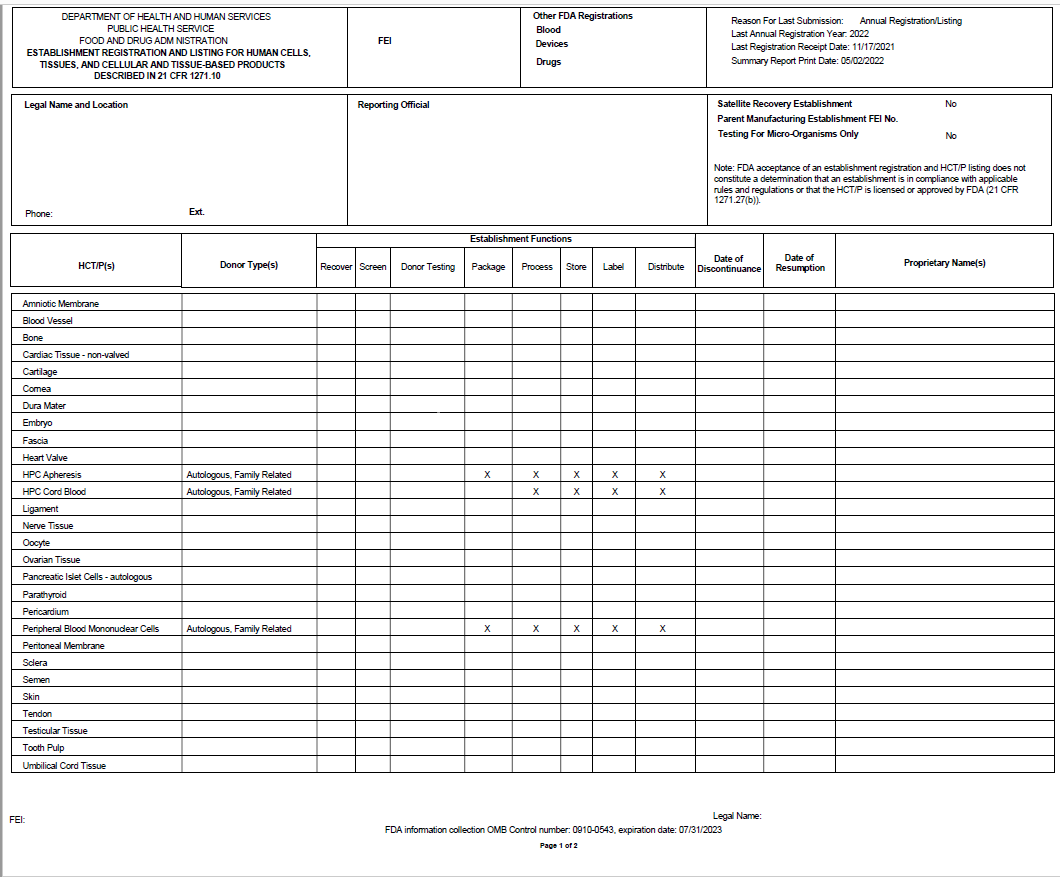
Registration Summary Report Provided to the Establishments (Example-Page 2)

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Bullock, Monica-Tia |
| File Modified | 0000-00-00 |
| File Created | 2023-08-30 |
© 2026 OMB.report | Privacy Policy