May 2021 CHANGE REQUEST ALS Biorepository Protocol with COVID-19 Restart Plan (clean)
Appendix S - ALSBiorepository_Protocol-COVID-19 Restart Plan v1 clean.docx
National Amyotrophic Lateral Sclerosis (ALS) Registry
May 2021 CHANGE REQUEST ALS Biorepository Protocol with COVID-19 Restart Plan (clean)
OMB: 0923-0041
Appendix S National ALS Biorepository Version 1 – Amendment 6
|
|
|
|
|
|
Table of Contents
Introduction 4
Background 4
ALS Epidemiology and Genetics 4
ALS Biomarkers 6
ALS Biorepositories 6
Best Practices for Biorepositories 9
Anticipating Research Uses 10
Pilot Study Results 11
Approach 13
In-home Biorepository Component 14
Selecting prospective participants 14
Enrolling participants 15
Preparing for specimen collection 16
Collecting specimens 17
After specimens are collected 18
Event collection Biorepository Component 19
Postmortem Biorepository Component 19
Selecting prospective participants 19
Enrolling participants 19
Preparing for specimen collection 20
Collecting specimens 22
After specimens are collected 22
Human Subjects Considerations 23
Distribution of specimens 24
Specimen Analysis Conducted by the National ALS Registry 24
Results of research 26
References 27
Tables 30
Table 1. Factors previously studied for association with ALS, with selected examples citing relevant biomarkers of exposure 30
Table 2. Frequencies of mutations in five genes associated with familial ALS in a study of 162
families 31
Table 3. Pathophysiologic processes with hypothesized roles in ALS, with selected examples citing approaches to measurement 32
Table 4. Proposed desirable minimum clinical dataset for ALS biomarker studies 33
Table 5. Biorepositories and brain banks with samples from people with ALS 34
Table 6. UK Biobank specimen collection protocol 35
Table 7. Relative advantages of different specimen types for biomarker research 36
Table 8. Proposed minimum specimen collection protocol 37
Figures 38
Figure 1. Genetic association studies of sporadic ALS, by year, 2001-2011 38
Appendices 39
Appendix A: Request for additional information 39
Appendix B: In-home Component 40
Appendix B-1. Communications 40
B-1a. Email/letter to those expressing interest in biorepository 40
B-1a(1). Email/letter (full collection) 40
B-1a(2). Email/letter (saliva only) 42
B-1b. Phone script to schedule appointment, review instructions, and obtain consent 44
B-1c. Letter/email appointment confirmation and instructions 47
B-1c.1 Letter/email appointment confirmation and instructions for just a blood draw 48
B-1d. Phone script to reschedule appointment 49
B-1e. Email/letter blood draw failure 51
B-1f. Phone script for failed blood draw or unusable blood 52
B-1g. Email/letter for saliva collection 54
B-1h. Phone script follow-up saliva collection 55
B-1i. Email/letter thank you for participating in the biorepository 56
B-1j. Phone script to provide additional blood (and other specimens) collection options……………... 57
B-1k. Phone script for when a participant who has provided a saliva specimen is contacted again to collect blood (and other specimens)………………………………………………………………………………………………… 60
B-1l. Phone script for when a potential participant has agreed to a blood (and other specimens) collection is contacted again but no collection took place………………………………………………………………… 61
Appendix B-2. Other participant materials 62
B-2a. Fact Sheets 62
B-2a(1). Fact Sheet Full Collection 62
B-2a(2). Fact Sheet without Hair and Nails 65
B-2a(3). Fact Sheet Saliva only 67
B-2b. Instructions for opening the specimen collection kit 69
B-2b.1 Instructions for opening the specimen collection kit when not collecting urine 70
B-2c. Instructions for participant urine collection 71
B-2d. Instructions for saliva collection 72
Appendix B-3. Consent forms 73 B-3a. In-home collection (biospecimens) 73
B-3b. In-home collection (saliva) 77
Appendix B-4. In-home specimen collection procedures 80
B-4a. Urine 80
B-4b. Blood 81
B-4c. Hair 82
B-4d. Fingernails 83
B-4e. Specimen Processing Form 84
Appendix B-5. Email/letter announcement event collections 86
Appendix B-6. Biorepository Safety Information………………….………………………..................................... 87
B-6a. Biorepository COVID-19 Safety Procedures and Restart Plan….…………….…………………………..... 87
B-6b. National ALS Biorepository COVID-19 Safety Information Sheet………………………………….......... 91
Appendix C: Postmortem Component 92
Appendix C-1. Communications – Postmortem component 92
C-1a. Email/letter to those interested in biorepository 92
C-1b. Phone script to begin eligibility screening 94
C-1c. Phone script to provide eligibility screening update 96
C-1d. Phone script to inform potential participants that physician is not responding 98
C-1e. Phone script to notify ineligible participants 99
C-1f. Phone script to schedule consent appointment 100
C-1g. Email/letter to confirm appointment 102
C-1h. Phone script to obtain consent 103
C-1i. Email/letter to those who have not agreed to participate 105
C-1j. Phone script to contact those who have not agreed to participate 106
C-1k. Email/letter thank you for agreeing to participate 108
C-1l. Email/letter thank you and Donation Plan 109
C-1m. Phone script to get health status updates 111
C-1n. Email/letter thank you for family 112
C-1o. Email/letter thank you for family where donation failed 113
Appendix C-2. Other participant materials – Postmortem component 114
C-2a. Fact Sheet 114
C-2b. HIPAA Authorization form 116
C-2c. Eligibility Checklist 118
C-2d. Family Authorization form 119
Appendix C-3. Consent form – Postmortem component 120
C-3a. Consent Form Addendum 125
Appendix C-4. Postmortem specimen collection procedures 127
C-4a. Brain, spinal cord, and CSF 127
C-4b. Muscle and bone 128
C-4c. Postmortem collection of skin specimens 129
Appendix C-5. Processing of brain, spinal cord, and CSF specimens 130
Appendix C-6. Neuropathology data collection form 133
Introduction
No single study or database can provide answers to the most pressing questions about ALS, such as: How many people have ALS? What are the underlying causes? How can understanding these causes lead to prevention and treatment? What biomarkers are useful for predicting disease progression and treatment response? Answering such questions requires the integration of epidemiologic, clinical, and basic research findings to place individual measurements in context, suggest hypotheses, and provide a basis for inference. This biorepository will enhance the Registry’s utility for research on ALS. It will differ from existing biorepositories because it is population-based and nationally comprehensive, rather than defined by geographic area, exposure, or clinical characteristics. The National ALS Biorepository will increase the number of biological samples available for research and will make them available to ALS researchers regardless of institutional affiliation. Persons with ALS have expressed interest in providing biological specimens through the National ALS Registry.
Background
ALS Epidemiology and Genetics
ALS occurs worldwide but because of its rarity, variation and trends in ALS incidence are difficult to measure. The only consistently identified risk factors for ALS are increasing age and male sex. Although tobacco smoking has been proposed as an additional “established risk factor” (Armon 2009), findings among studies are inconsistent and the effect on risk (if any) is small (Alonso 2010). Epidemiologic studies have examined many other potential risk factors for ALS, including occupation, trauma, infections, exposure to metals and other chemicals, and electric shock (Table 1). Potential biomarkers have been proposed for some of these exposures or their pathologic sequelae; however, so far few epidemiologic studies have incorporated biomarkers.
Like other degenerative neurologic diseases, including Alzheimer’s and Parkinson’s diseases, ALS is thought to develop as a result of complex interactions among multiple genetic and environmental risk factors (Ahmed 2011; Siddique 2011). The clinical heterogeneity of ALS suggests that different underlying mechanisms and pathways could be involved.
Familial ALS. Approximately 10% of people diagnosed with ALS have an affected first-degree relative (Fang 2009; Siddique 2011). Fang et al. conducted a retrospective cohort study of familial ALS by analyzing the Swedish Multi-Generation Register from 1961 to 2009. They found that the risk of ALS was increased about 10-fold in siblings and children of affected persons; risk was further increased if the proband was diagnosed at a younger age. Clustering of ALS within families suggests that genetic factors contribute to susceptibility.
Beginning in the 1990’s, genetic studies of families with multiple affected members implicated mutations in several genes that typically confer risk in an autosomal dominant manner, although autosomal recessive inheritance has been observed in some families (OMIM, 2012). Variations in superoxide dismutase 1 (SOD1) were first associated with familial ALS in 1993; since then, more than 130 SOD1 mutations have been implicated, accounting for approximately 10–20% of multiply affected ALS families (Millecamps 2010).
A recent French study of 162 distinct families with ALS examined the frequencies of mutations in five previously associated genes; results are summarized in Table 2 (Millecamps 2010). A mutation in at least one of these five genes was found in only 36 (22%) of the 162 families; of the 31 distinct mutations identified, 7 (23%) had not been previously reported. The finding of largely private mutations in multiple genes attests to the heterogeneity of genetic susceptibility to ALS.
Recently, two studies implicated expansion of a GGGGCC hexanucleotide repeat in C9ORF72, a non-coding region of chromosome 9, in families of European ancestry affected by both ALS and frontotemporal dementia (FTD) (DeJesus-Hernandez 2011; Renton 2011). DeJesus-Hernandez et al. found this repeat expansion in 22.5% of familial ALS seen in three clinics (Mayo Clinic, University of British Columbia, and University of California—San Francisco). Renton et al. found the same expansion in nearly half of all familial ALS in Finland and in one-third of 268 familial ALS probands of European ancestry.
The ALS Online Database (ALSoD, http://alsod.iop.kcl.ac.uk), an open source data repository for sharing ALS genotype and phenotype data, was developed by a consortium within the World Federation of Neurology (Lill 2011). Consortium members submit their own data and extract data from scientific publications; users of the database can also upload their own data. The focus is on familial ALS, although data from non-familial cases are also accepted. As of January 2012, the database included more than 450 records for people with familial ALS; mutations in 17 genes were represented, including the five studied by Millecamps.
Discoveries of additional mutations in families affected by ALS continue to offer clues to underlying disease processes. For example, Wu et al. in 2012 used exome sequencing in families with ALS to discover associated mutations within the profilin 1 (PFN1) gene.
Sporadic ALS. New technologies and information stemming from the Human Genome Project have spurred genetic association studies of “sporadic” ALS in persons without a family history (Figure 1). These population case-control studies are the focus of the ALSGene database (www.alsgene.org), which as of January 2012 included more than 250 polymorphisms in more than 90 genes, including some previously identified in family studies (Lill 2011).
In genetic association studies of sporadic ALS, observed odds ratios are typically small (between 1.1 and 2.0), even for variants of genes implicated in familial ALS. Such findings are typical for genetic association studies of multifactorial chronic diseases, including atherosclerosis and cancer. No genetic association with sporadic ALS has been found to have effects as large and consistent as the association of APOE genotype with Alzheimer disease (meta-analysis odds ratio [OR] 3.68, 95% CI 3.30–4.11, www.alzgene.org, January 2012) and even strong genetic associations serve only as the starting point for further investigations of pathogenesis (Storandt 2012). Since 2005, genome-wide association studies (GWAS) have been a powerful tool for identifying new candidate genes in complex diseases. The results for GWAS of ALS have offered only a few leads and replication of the promising association with DPP6 has been inconsistent (Chio 2009).
ALS Biomarkers
Advances in molecular biology and neuroimaging technologies are providing an increasingly detailed picture of neuropathology in ALS. Biomarkers of contributory exposures, disturbed physiologic processes, and tissue damage may be useful in discovering underlying physiologic mechanisms. Several proposed mechanisms and categories of potential biomarkers are summarized in Table 3. Many of these have been identified in basic research using neural tissue obtained postmortem.
A vital goal of pathophysiologic research is to identify biomarkers that can be used clinically, to diagnose ALS earlier and reduce diagnostic delay, predict disease progression, stratify patient populations for clinical trials, and assess response to treatment (Bowser, 2011, Otto 2012). Desirable attributes of clinical biomarkers include their presence in accessible specimens (e.g., blood, urine); detectability early in disease; high sensitivity and specificity; and high predictive value for relevant clinical outcomes. The successful development of such biomarkers requires integrating findings from epidemiologic, clinical, and basic science research.
A recently published article, “Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS,” followed from a 2010 workshop sponsored by the World Federation of Neurology (Otto 2012). This report systematically describes key considerations in the design and conduct of research on ALS biomarkers and the need for international collaboration and standardization. The authors point out that “biomarker studies are of limited or no use without reliable clinical characterization of patients and propose a “desirable minimum clinical dataset,” which is included as Table 4 of this protocol.
Bowser et al. (2011) recently reviewed candidate protein-based, neurophysiological, and neuroimaging biomarkers in ALS. Although all three types of biomarkers are potentially measurable in clinical settings, only protein-based biomarkers will be available from samples collected for the National ALS Biorepository. Candidate protein-based biomarkers include various molecules with a role in innate immunity (e.g., interleukins, chemokines), complement factors, and markers of neural tissue damage. Quantitative methods for measuring candidate protein biomarkers include antibody-based methods (quantitative ELISA, multiplex antibody capture) and various combinations of protein purification procedures (gel electrophoresis, liquid chromatography) with mass spectrometry.
The discovery and validation of biomarkers is an area of ongoing, active research. As of January 2012, the NIH clinical trials database (http://clinicaltrials.gov/) included at least 18 studies of ALS that included collection of biomarkers, of which four were actively recruiting. For example, enrollment began in November 2011 for a new longitudinal study of ALS biomarkers (NCT01495390) at Massachusetts General Hospital.
ALS Biorepositories
Understanding the complex pathobiology of ALS, which appears to involve multiple complex, interdependent processes over time, requires the capacity to detect factors with small effects on overall risk. Thus, there is a strong incentive for ALS clinical researchers to collaborate in establishing standardized diagnostic criteria (such as the El Escorial criteria, first published in 1994) and frameworks for sharing clinical data and biological research specimens.
Many ALS clinical registries have been established at academic and other research centers; typically, these registries consist of data and specimens collected from patients at the time of diagnosis and treatment or during the course of clinical trials. The data and specimens may be shared in ad hoc research collaborations. ARISLA, an Italian foundation for research on ALS, maintains a list of European and U.S. biobanks and repositories (with brief descriptions and links) at http://www.alscience.it/?view=52#v52.
Tissue banks containing postmortem specimens of brain, spinal cord, and muscle tissue are also important resources for ALS research. The International Brain Banking Network (IBBN) (http://www.intbbn.org/) facilitates communication among brain banks and conducts annual workshops in association with the American Association of Neuropathologists. The IBBN website includes a directory of brain banks in the U.S. and other countries (http://www.intbbn.org/registry-of-brain-banks.aspx); most of these banks are at universities and many have an Alzheimer research focus.
Several independent organizations offer specimen retrieval services that will query registries and tissue banks for specimens with specific characteristics and request them for use by outside researchers. For example, the National Disease Research Interchange (http://www.ndriresource.org) is a not-for-profit corporation funded mostly by NIH that locates biological research materials and maintains a specimen bank for the NIH Office of Rare Diseases Research (ORDR, http://biospecimens.ordr.info.nih.gov/). SpecimenCentral.com (http://specimencentral.com/) is a commercial broker, connecting researchers with biobanks holding the types of biological samples they are seeking.
Prospective collaborations in the US and elsewhere have developed clinical research resources for ALS, including biospecimen collections. The principal examples are summarized briefly below and in Table 5.
New England ALS (NEALS) Consortium. The NEALS Consortium is a network for ALS clinical research that was formed in 1994 and has grown to more than 90 members. NEALS provides infrastructure for multi-center clinical trials and other clinical research studies. The Massachusetts General Hospital (MGH) serves as the Coordinating Center for many of these studies and houses an associated biofluid repository containing a variety of blood, urine, plasma, cerebrospinal fluid (CSF), and extracted DNA samples. Biorepository samples are linked to clinical data and stored under controlled conditions, providing potentially larger sample sizes for research than are available from single studies. The NEALS Sample Sharing Committee oversees sample sharing policies and reviews data and sample requests. A scalable “virtual biobank” platform has been developed to allow investigators in ALS clinical research centers to share and search for samples while retaining control of their own specimens and data (Sherman 2011).
National Institute of Neurologic Diseases and Stroke (NINDS), NIH. In 2004, NINDS added ALS to the conditions included in a DNA bank and cell line repository established at the Coriell Institute to support large-scale research on the genetics of neurologic diseases. This effort was a public-private collaboration of NINDS with the ALS Association (ALSA), the Muscular Dystrophy Association (MDA), and academic researchers from 62 centers across the United States, including many members of the ALS Research Group (http://www.alsrg.org) (Gwinn 2007). The repository is a national research resource of biological samples linked to individual phenotypic datasets. For all included subjects, NINDS Clinical Data Elements (CDE) must be complete; these include demographic, clinical, and medical and family history data (Gwinn 2007, Appendix S1B). With the exception of smoking (optional), data on potential environmental exposures are not collected. The repository includes specimens from approximately 2000 people with ALS, including blood, immortalized lymphocytes, and fibroblasts. Some samples from unaffected and affected blood relatives of subjects, spouses, and healthy individuals (including population and convenience controls) are also included. The repository is not actively adding specimens, although researchers may apply to submit their own collections. More information is available at http://ccr.coriell.org/Sections/Collections/NINDS/Motor.aspx?PgId=192&coll=ND.
Medical Research Council (MRC) London Brain Bank for Neurodegenerative Diseases. The MRC London Brain Bank was established in 1989 in the Department of Neuropathology, Institute of Psychiatry, King’s College London, UK, to make clinically and pathologically well-characterized brain and spinal cord tissue available to researchers worldwide (http://www.iop.kcl.ac.uk/departments/?locator=380). The MRC London Brain Bank focuses on neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, and motor neuron disease (including ALS). Currently, specimens are available from 189 persons with motor neuron disease and the bank maintains an ongoing program to “facilitate brain donation through an ethically approved program of informed consent for cohort studies and ad-hoc donations.”
Several epidemiologic studies of ALS have used population-based registries to identify potential participants, who are contacted to request biological specimens (Chio 2009). Very few population-based ALS registries have been designed to collect specimens prospectively. In the US, the only large population-based ALS registry with an associated biorepository is the National Registry of Veterans with ALS. This biorepository includes blood (or buccal cell) specimens collected for DNA analysis, as well as a brain bank; because it offers the most relevant model for development of the National ALS Biorepository, it is described in additional detail here.
National Registry of Veterans with ALS. The registry was established by the Veterans Administration (VA) after increased incidence of ALS was reported in veterans deployed to the Persian Gulf in 1990–91 (Kasarskis 2004). The objectives of the registry were to identify living US military veterans with ALS, follow their health status, collect data for epidemiologic research, and inform veterans about clinical trials for which they could be eligible (Allen 2008). Veterans with ALS were identified from VA medical databases and via nationwide solicitation; a brief telephone interview screened potential participants for eligibility. During the enrollment period (April 2003–September 2007) more than 2,000 participants aged 23–93 years were enrolled, spanning combat eras from World War II to the 1990–91Gulf War.
Registrants were asked to provide contact information for all providers of healthcare since their onset of ALS symptoms. Their medical records were retrieved and abstracted by trained personnel, who recorded results of physical and electromyographic examinations, pulmonary function tests, and laboratory and imaging studies, as well as medical history (including surgery and physical trauma) and family history of ALS. An integral component of the registry is the DNA bank, which is a collaboration of the ALS Registry (Epidemiologic Research and Information Center, Durham VAMC), the VA DNA Coordinating Center (Palo Alto VAMC), and the Genetic Tissue Core Laboratory at the Massachusetts Veterans Epidemiology Research and Information Center (Boston VAMC). All registry participants were asked to contribute a specimen, although it was not required. DNA specimens were collected in participants’ homes by trained nurses associated with a nationwide home health agency. Nurses were supplied with blood collection kits, as well as mouthwash for use in collecting buccal cells when no blood specimen could be obtained. Of the more than 2,000 veterans enrolled in the registry, 76% provided written consent for participation in the DNA bank; 8% refused and the remainder were excluded for other reasons. More than 1,000 specimens were obtained, of which 85% were blood and 15% were buccal cells only.
A brain bank component was added to the registry in 2006. The VAB Brain Bank is coordinated at the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) at VA Boston Healthcare System (VABHS). Tissue is analyzed, processed, and stored at the Southern Arizona Core Tissue Laboratory (SACTL) at the Southern Arizona VA Healthcare System (SAVAHCS) in Tucson, AZ. Although enrollment in the registry ended in 2007, regular telephone follow-up of enrolled Veterans has been continued by the VAB. This follow-up includes semi-annual administration of the ALS Functional Rating Scale. Postmortem collection of brain tissue continues for previously enrolled participants. Additionally, the VAB continues to enroll Veterans with ALS via referrals from the Neurology Service at VA Boston, and other VAs. A Scientific Review Committee provides expert review for all requests to use registry data and samples. Multiple studies have already been conducted on a range of topics, including genetics, environmental exposures, disease progression, psychosocial issues, and a brain-computer interface for patients (Allen 2008). The National Registry of Veterans with ALS is also the source of cases for the GENEVA Study, a case-control study of genes, environmental exposures, and gene-environment interactions in ALS (Schmidt 2008; Schmidt 2010). The VAB has begun to release tissue and associated data to investigators.
Best Practices for Biorepositories
International Society for Biological and Environmental Repositories (ISBER). ISBER publishes best practices for the collection, storage, retrieval and distribution of biological materials for research. The second and most recent edition was published in 2012 and is available online at https://c.ymcdn.com/sites/www.isber.org/resource/resmgr/Files/ISBER_Best_Practices_3rd_Edi.pdf (ISBER 2012). Guidelines in this document address all aspects of biorepository management, including administration and records management, facilities and equipment, safety, quality assurance, specimen tracking and management, and ethical and legal issues, as well as best practices for human biospecimen collection, transport, and storage. A key point notes the value of pilot or feasibility studies for identifying problems in specimen collection and handling before beginning a larger study.
National Cancer Institute (NCI). NCI has invested in extensive biorepository programs to support basic, epidemiologic, and clinical research on cancer. Recognizing the increased value and changing uses of biospecimens in clinical research, NCI commissioned the National Biospecimen Network Blueprint (2003), which recommends ways to enhance the collection of biospecimens and the management of biorepositories. The NCI Clinical Trials Cooperative Group Program, which includes more than 3,000 institutions and more than 14,000 investigators, also maintains the network of Cooperative Group Banks (http://cgb.cancer.gov/). Banked biospecimens include formalin-fixed, paraffin-embedded tumor and normal tissue, fresh frozen tumor and normal tissue, blood, serum, plasma, urine, genomic DNA, and bone marrow. A directory of participating Cooperative Group Banks is available online at http://cgb.cancer.gov/contacts/index.html.
In 2006, NCI’s Office of Biorepositories and Biospecimen Research (OBBR) established the Biospecimen Research Network to assess the effects of pre-analytical factors (e.g., specimen types, collection methods, transport, processing, and storage) on the outcomes of genomic and proteomic studies in cancer research. OBBR has issued Best Practices for Biospecimen Resources that address scientific and technical considerations, as well as ethical and legal issues. Last updated in 2011, these Best Practices are available online at http://biospecimens.cancer.gov/bestpractices.
Public Population Project in Genomics (P3G). P3G is an international non-profit organization that promotes collaboration among researchers in population-based genomic research. The P3G Observatory (http://www.p3gobservatory.org/) is an online repository of information and tools for harmonization among individual biobanks. The Observatory includes a directory of relevant guidelines and best practices at http://www.p3gobservatory.org/repository/sampleCollection.htm. In addition to the ISBER and NCI guidelines already mentioned, these include Best Practice Guidelines for Biological Resource Centers published by the Organization for Economic Co-operation and Development (OECD) and other national and international research organizations.
Anticipating Research Uses
Anticipating future uses of biorepository samples is challenging, especially because technology continues to expand the realm of possible analyses. Although versatile specimens—such as whole blood, serum, and plasma—are likely to be most useful in the long run, collecting specimens for future use always requires trade-offs: for example, between collecting a larger variety and volume of specimens vs. cost of collection and storage, or between asking participants to consent to unspecified future analyses vs. their willingness to participate.
Choosing the types and quantities of specimens to be collected for a biorepository depends on multiple considerations. A recently published biobanking “roadmap” for ALS biomarker research compared the advantages and disadvantages of several types of specimens, which are summarized in Table 7 (Otto 2012). In addition to blood and cerebrospinal fluid (CSF), which have been studied most often, the roadmap considered urine, saliva, skin, and muscle tissue. Urine and saliva offer practical advantages relative to blood: collection procedures are less invasive and analysis is simpler (although also more limited) because of their less complex molecular composition. Urine has potential added value as a specimen for measuring levels of drugs and other environmental chemicals and their metabolites. Saliva provides an alternative source of DNA when blood collection is not possible. CSF, skin, and muscle tissues can only be collected in a clinical setting. Nail and hair clippings can provide information about past environmental exposures (such as those in Table 1), mostly limited to near- and intermediate-term exposures (Goullé 2009).
Whole blood remains the preferred specimen type for biobanks intended to serve as long-term research resources. Its advantages include:
DNA yield (quality and quantity);
versatility (e.g., for analysis of RNA and other biomolecules, as well as markers of environmental exposures);
straightforward creation of aliquots for sample sharing;
long-term stability in storage; and
potential for creating cell lines.
Whole blood specimens must be collected with the intended analyses in mind to prevent interference by anticoagulants. For example, collecting blood in anticoagulant-containing tubes causes the release of cytokines, resulting in spuriously elevated concentrations. Whole blood specimens collected on filter paper (Guthrie cards) share many of these advantages with conventional blood specimens; however, their small volume limits the numbers and types of possible analyses and they can’t be used to establish cell lines.
Pilot Study Results
Through a contract ATSDR conducted a study to pilot methods for collecting and banking biological specimens from participants in ATSDR’s National ALS Registry. The Pilot Study included two specimen collection components: biological specimens from living participants (in-home) and postmortem specimens. The in-home component enrolled 330 participants, from whom specimens were collected on two occasions, approximately six months apart. The postmortem component enrolled 30 participants, who could also participate in the in-home study. In-home collection included blood, urine, hair, and nails. The postmortem collection included the collection of brain, spinal cord, CSF, muscle, bone, and skin specimens for the creation of cell lines.
Persons participating in the in-home collection were asked to provide consent over the phone. Those participating in the postmortem collection were visited in their homes and asked to provide consent in person. When skin specimens were added to the tissue types collected postmortem, a consent addendum was created and approved by the IRB. Participants who provided consent to participate before the addition of skin specimens were contacted about participating in this additional specimen type and asked to provide consent over the phone. For all parts of the consent process that took place over the phone, the participant returned a signed copy of the consent form to McKing before any study procedures took place. Participants were provided a copy of the signed consent form for their records.
Three hundred and thirty-nine individuals provided consent to take part in the Pilot Study, 202 (59.5%) male and 137 (40.5%) female. Of the 339 biospecimen participants, 221 (64.8%) were recruited and 118 (35.2%) were volunteers. Nine of the 339 participants were unable to schedule an appointment, resulting in 330 participants completing at least one specimen collection. Thirty individuals provided consent to take part in the postmortem part of the study. Of the 30 postmortem participants, 5 (16.7) were recruited and 25 (83.3) were volunteers. All 30 postmortem participants provided consent for the overall donation of brain, spinal cord, bone, muscle, and CSF. In addition, 27 of the 30 the postmortem participants provided consent for skin sample collection. Three participants did not provide consent for skin sample collection (one died before skin sample collection was added to the protocol, one refused, and one died before the signed skin consent form was received).
There were 330 participants who completed first specimen collections. Three hundred and eleven participants provided at least one vial of blood and 19 were unable to provide any blood for the first specimen collection.
There were 272 participants that completed second specimen collections. Two hundred fifty-five provided at least one vial of blood and 17 were unable to provide any blood during the second specimen collection. Fifty–eight of the participants who had a first specimen collection did not have a second specimen collection. The reasons for not completing the second draw included death (36), too ill or unable to contact (9), and no longer interested or scheduling difficulties.
Three hundred and twenty-one participants provided at least one blood specimen and 15 provided a saliva sample. Nine participants were unable to provide any blood; however, eight provided hair samples, nine provided nail samples, nine provided a urine sample, and four provided a saliva sample.
Most (78%) participants were able to provide specimens at both collection appointments.
Eighteen postmortem participants have donated specimens as of November 12, 2015. The length of time in the study for these participants from date of consent to date of death ranged from 1-24 months and the length of time in the study for participants that are still living, ranged from 20-27 months. The age at death for the deceased participants ranged from 43-76 years of age. Two participants are deceased but withdrew from the postmortem part of the study and did not provide postmortem donations. However, these participants did take part in the biospecimen part of the study.
Creating a geographically-diverse biorepository had unique challenges. Recruitment was slower than expected, finding reliable phlebotomists across the country was difficult, and there were unexpected issues related to shipping specimens including higher than average temperatures and mechanical failure. In addition, after the first specimens were collected there was a larger than expected number of individuals that were too sick or deceased and could not participate in the second specimen collection, decreasing the number of paired specimens. However, we were able to recruit the target sample size and process the varied specimen types. In March 2015, the Expert Panel extensively discussed the results of the pilot study and endorsed adding specimen collection as a component of the National ALS Registry. The Expert panel felt that the specific specimens collected should remain flexible so that there could be changes as research priorities and technology changed. In addition, they felt that because of the logistics and costs specimens should be collected at only one point in time. The discussion on the types of samples that should be collected as well as some changes to the logistics have been incorporated into the current proposal.
Approach
We will collect specimens from ALS patients enrolled in the National ALS Registry. The specimens to be collected will be determined on a yearly basis depending on the scientific demand. Currently, McKing is accessing demand by reviewing the literature and talking with key ALS researchers. In subsequent years, we will evaluate the actual researcher requests. For example, although hair and nails are easy to collect there is still a cost to the laboratory for inventory control and storage. Therefore, we may not collect any additional hair or nail samples until the ones from the pilot study have been used up. Specimen types that could be included for in-home collection are blood, urine, hair, and nails. The amount of blood collected will not exceed 50 ml. Types of blood specimens could include blood for serum and DNA, blood for plasma, metals free collection of blood, blood for RNA extraction, and blood to create cell lines. For the postmortem collection, we will collect the brain, spinal cord, CSF, bone, muscle, and skin.
Population. Eligible participants for either component must have enrolled in the congressionally mandated National ALS Registry. After an individual has joined the Registry he/she will have the opportunity to ask for additional information about the biorepository and provide needed contact information (Appendix A). Additional specific eligibility requirements are outlined for each component below.
In-home component. Specimens will primarily be collected in participants’ homes by trained phlebotomists. This was the process used by the biorepository pilot study and VA ALS Registry specimen collection. This approach is more convenient for participants and less likely to discourage those whose mobility is severely restricted. Minimizing limitations on when or where specimen collection can take place improves participation.
During the pandemic, the Registry paused Biorepository specimen collections to protect the health of the Biorepository participants and members of their household. A detailed restart safety plan has been added (Appendix B-6a).
It is possible that ATSDR will want to collect blood specimens that need immediate processing and special handling. For example, some analytic techniques require plasma samples that have been centrifuged within 15 minutes of collection and then frozen. If this is the case, we will arrange for some participants to come to central locations for these specialized collections.
The phlebotomist will use a commercially available kit to collect the specimens and will be responsible for sending collected specimens to the laboratory according to the shipping protocol. Practical issues preclude the collection of specimen types that require a medical procedure or immediate processing (e.g., skin or muscle biopsy, lumbar puncture for CSF). Table 8 indicates minimum specimen collection requirements, as well as potential additional specimens that could be self-collected and shipped by the participant or caregiver.
In-home saliva only component. DNA is one of the most used specimen types as more and more genes associated with ALS are identified. In order to increase the number and diversity of DNA specimens available for analysis, we will collect only saliva from an additional group of ALS patients enrolled in the Registry. We will sent a salvia collection kit to the participant’s home with instructions for collection along with a pre-paid shipping label to return it to the lab.
Postmortem component. Research on the pathobiology of ALS has naturally emphasized analysis of brain, spinal cord, and CSF, the tissues most proximal to the disease process. The Boston University Alzheimer's Disease Center Brain Bank has developed procedures based on ISBER best practices (ISBER 2012) to recover these tissues postmortem. Their procedures will be used for the postmortem component of the biorepository with additions to include collection of muscle and bone tissue specimens.
Sample size. The number of participants will vary from year to year depending on funding. The National ALS Biorepository would like to collect a full set of biological specimens from 250 – 325 participants per year. Based on the blood failure rate in the pilot study, we anticipate that about 10% of the participants will be unable to give blood and will be offered the opportunity to provide a saliva sample. In addition, we will collect saliva only on 350 participants per year. Those who provide saliva only will be a combination of those who were unable to provide a blood specimen and those selected for saliva only collection. Therefore in a year with 325 participants in the full biological specimen component and 350 saliva only collections, we are expecting 675 participants. We would like to obtain postmortem tissue from 40 participants, i.e., approximately 10 per year.
Training staff. All biorepository staff will be trained on biorepository procedures and data security by the Senior Scientist. All biorepository staff will be required to provide documentation of training in HIPAA or take a certified course in HIPAA regulations that pertains to research. All biorepository staff will also take approved Human Subjects Protection training.
Outreach. Staff from the National ALS Biorepository will attend ALS related events (e.g., ALS Walks and ALS Association meetings) to answer questions about the project and handout IRB approved materials such as the factsheet and introductory letter. These activities will increase the biorepository visibility and better establish the connection with the National ALS Registry.
In-home Biorepository Component
Selecting prospective participants
Eligibility. To be eligible to donate specimens, prospective participants must have enrolled in the National ALS Registry and requested additional information about the biorepository
Identifying prospective participants. To achieve the target sample size of 675 participants per year, we estimate that it will be necessary to contact approximately 325-350 persons enrolled in the National ALS Registry for the full collection and 350-400 for saliva only collection. After providing consent to take part in the Registry, a potential participant will be asked if he/she would like to more information about the Biorepository (Appendix A). On a monthly basis, ATSDR will provide a list of those in the National ALS Registry who have expressed an interest in the biorepository. This list will include the date of birth, sex, current address, telephone number, and date of diagnosis for each registrant. Each month individuals will be selected to receive an invitation to participate in the biorepository based on geographic area. If a person with ALS calls and wants to provide specimens for the biorepository, he/she will be provided information on how to join the Registry and indicate interest in the biorepository.
Selection full collection and saliva. The number of participants to be recruited each month will be determined by the number for the entire year. Each month ATSDR will provide McKing with a listing of Registry participants interested in the biorepository. To better address the congressional mandate to examine genetic and environmental risk factors that may cluster by geographic area, we will select a convenience sample from those who are interested in the biorepository proportional to state population and with at least one person from each state. We will recruit from the harder to fill states, e.g., Wyoming, Rhode Island, first and then distribute the cases throughout the other states. Because recruitment tends to get individuals from the same town to enroll during the same time period, selection in states where we are recruiting multiple participants will be distributed across the states in any given month. Based on our experience with the pilot study, this will give a good distribution of those living in urban and rural areas in addition to good state representation. At the time of selection, potential participants will be assigned to full collection or saliva only and receive the appropriate recruitment materials and consent form.
Demographic composition of biorepository population. A spreadsheet will be developed to track recruitment which includes age, sex, city, and state. We will attempt to assure the participants are diverse as possible within those participating in the National ALS Registry. After each round of participant selection, biorepository staff will examine the distribution of enrolled participants by geographic area, age, and sex, so that subsequent recruitment can target enrollment of the underrepresented groups. We will determine the number of individuals that should be recruited per state based on state population.
Enrolling participants
Recruitment. A sample of persons indicating an interest in the biorepository (full collection or saliva only) will be mailed a packet of information that includes an introductory letter (Appendix B-1a(1) or B-1a(2)), factsheet (Appendix B-2a(1) or B-2a(2)), and consent form (Appendix B-3a or B-3b). Approximately one week (5-10 days) after the packet is mailed, McKing biorepository staff will contact the potential participant to determine if he/she has received the packet, answer any questions, and if interested in participating, go over the consent form. The consent form will be signed, by the participant or witness, at the time of the phone call and returned in a self-addressed stamped envelope. The witness is just attesting that the consent form was explained to the participant and he/she agreed. Only the participant can provide consent. If a participant decides after reviewing the consent form that he/she does not want to participate, the individual will be removed from future contact lists for the biorepository. We will attempt to reach the person by phone up to 3 times.
Scheduling specimen collection. McKing biorepository staff will answer all questions about the biorepository and maintain all direct contact with participants except for collecting the specimens. Each prospective participant who verbally confirms his/her willingness to participate will be offered a range of dates and times to schedule a visit by a phlebotomist to collect the specimens (Appendix B-1b). We anticipate that most participants will choose to have specimens collected at their place of residence; however, acceptable alternative locations will be found for those who prefer for specimen collection to occur elsewhere. Biorepository staff will assign a unique identification number (Biorepository ID) to each participant at the time his/her first appointment for specimen collection is scheduled.
Within one week after scheduling an appointment for specimen collection, biorepository staff will send the participant a confirmation letter with the appointment location, date, and time (Appendix B-1c). The letter will include and instructions for how to open the specimen collection kit to remove the urine collection portion (Appendix B-2b). If the participant is only providing a saliva sample, a kit will be mailed within one week of receiving the signed consent form.
No appointment will be scheduled for the saliva only collection.
Preparing for specimen collection
Alternative collection arrangements
Because of the pandemic we are unable to send phlebotomists to participants’ houses. Therefore, we would like to allow home health care workers and physicians to draw the bloods. This would only be done at regularly scheduled doctor’s visits or with home health care workers already employed by the participant. This will ensure no additional potential exposures to coronavirus. McKing Biorepository staff will confirm qualifications to draw blood such as a nursing license or Certification of Phlebotomy Training (CPT).
In addition, while the Biorepository usually collects blood or saliva, there are some circumstances where we might want to collect both such as, but not limited to, the participant provided saliva when we were unable to collect blood, e.g., during the pandemic, or the participant had ALS related genetic findings where having blood would increase the usefulness of samples for research.
All other aspects related to recruitment, obtaining informed consent, kit shipment and specimen processing will remain the same.
Arranging the home visit. McKing has hired phlebotomy companies to provide phlebotomy services. They have the capability to provide nationwide specimen collection by hiring experienced local, licensed phlebotomists to collect specimens from participants who live in various regions of the U.S. McKing will provide information packets about the biorepository and collection requirements that will be provided to the contract phlebotomist. The phlebotomist will be provided via a secure method the name of the participant, date, time, address, phone number and location for specimen collection, at least one day prior to the scheduled in-home visit. The specimen collection kit, which includes detailed instructions for collecting the specimens, will be sent to the participant’s home.
The phlebotomist will contact the participant via telephone introduce herself and confirm the appointment for the next day. If there are any changes at this time, the phlebotomist contacts McKing to notify them of any changes. If the participant states on this call that he/she has decided against participating in the biorepository, the appointment will be cancelled and the participant will be removed from the biorepository contact list. Otherwise, the participant will be reminded about the procedures that will take place and given basic instructions related to eating and drinking before the specimen collection.
If the participant does not feel well enough on the day of specimen collection but still wants to participate in the biorepository, biorepository staff will reschedule the appointment (Appendix B-1d). The participant may call the McKing biorepository coordinator or notify the phlebotomist upon arrival at the participant’s home. Appointments will be rescheduled up to two times.
Specimen collection kits. Kits will be created that contain the necessary instructions and equipment for collecting each type of specimen (blood, urine, hair, nail clippings, saliva), as well as appropriate shipping containers with instructions for transporting specimens to the laboratory for processing. McKing will distribute and track kits mailed to the participants.
All materials shipped to and received back from the phlebotomist will be tagged with a barcode. The barcode will contain information about the type of sample and the Biorepository ID for each participant, which does not contain personally identifying information. A list of the barcodes and a data collection form will be included with each specimen shipment.
Collecting specimens
Detailed information on specimen collection and processing can be found in Appendix B-4.
Urine. Specimen collection will be a random or “spot” urine, because a 24-hour urine or timed urine is not feasible. Participants will receive instructions on how to open the specimen collection kit and remove the urine collection container (Appendix B-2b) before the visit from the phlebotomist with instructions on how to obtain the specimen (Appendix B-2c). Participants will be encouraged to collect the specimen in the morning prior to the appointment to obtain the first morning void.
Blood. Blood will be collected using a 21-gauge x ¾” multi-sample butterfly needle blood collection set, with 12” tubing and safety-lock for the needle. Specimens will be collected in a predefined order to minimize cross contamination of specimens.
Hair. Hair will be collected from the nape area of the head directly into the shipping container using scissors contained in the collection kit. Hair will not be collected from those who are bald or whose hair is too short.
Fingernails. Fingernails will be collected from all ten (10) fingers using a stainless steel nail clipper. Fingernail clippings will be collected directly into the shipping container. Fingernails will be collected from as many nails as possible. Fingernails will not be collected if they are too short. Toenails will not be collected because of possible circulation issues in the feet of ALS patients making it riskier than collecting fingernails.
Specimen processing form. Information necessary to process and interpret the results of the specimen analysis will be collected by the phlebotomist and shipped with the specimens (Appendix B-2e). The phlebotomist will have to ask the participant for some of the information required.
Saliva. Saliva will be collected using a self-collection kit from Oragene. The participant will be asked to spit into a small funnel mailed to his/her home. Instructions for providing the saliva specimen will be mailed with the kit. No additional information is collected with the saliva.
After specimens are collected
Shipping specimens to the laboratory. The phlebotomist will be responsible for shipping the specimens to the laboratory according to instructions included in the kit. The shipping containers provided in the kit are sufficient to maintain the contents at the correct temperature for at least 24 hours beyond anticipated time of arrival. To monitor temperature during transit, we will use multi-use temperature data loggers. These can provide continuous temperature data during transit. These are easy to use and allow the sites to activate them at time of shipment with the push of the button. The phlebotomist will take the package to the nearest FedEx manned drop-off and can call for shipment pick-up in remote areas if approved by McKing. The laboratory will notify biorepository staff via email when the shipment has been received.
Saliva will be shipped back to the laboratory by the participant. A self-addressed FedEx label will be included with collection kit along with directions on how to drop the box in a FedEx box or call for a FedEx pick-up.
Laboratory processing. The laboratory will aliquot, process, and store all specimens according to their standard operating procedures. Once inventoried, samples will be immediately placed into permanent locations and stored at the appropriate temperature for the sample type. The storage unit(s) will be monitored 24/7 by an electronic monitoring system in accordance with pre-approved temperature specifications.
Unsuccessful collection. If the blood draw fails during an in-home collection visit, the phlebotomist will notify biorepository staff. Biorepository staff will contact the participant and ask if he/she would like to provide a saliva specimen (Appendix B-1f). If yes, biorepository staff will send a self-collection saliva kit to the participant’s home (Appendix B-1e and Appendix B-2d).
If specimens become unusable after collection, biorepository staff will contact the participant, explain what happened, and ask if he/she are willing to provide a saliva specimen (Appendix B-1f). Once the participant agrees to provide a saliva specimen, biorepository staff will send a saliva self-collection kit and guidelines directly to the participant’s home (Appendix B-1g and Appendix B-2d). Biorepository staff will not ask participants to donate blood or urine again; however, if a participant offers to repeat the donation, biorepository staff will schedule a follow-up appointment for a phlebotomist to revisit the participant’s home at least 8 weeks after the first blood draw. Following completion of the biospecimen collection, a thank you letter will be mailed to each to thank them for taking part in the biorepository (Appendix B-1i). A repeat blood draw will only be considered for those where the blood was unusable due to shipping or laboratory error. Repeat blood draws will not be considered for failed blood draws.
Participant follow-up. Biorepository staff will send a thank-you note when specimens have been received (Appendix B-1i).
Event collection Biorepository Component
To increase participation in areas that are under-represented in the Biorepository, we will work with our partners, e.g., the ALS Association, to conduct specimen collections at events such support groups and symposiums. Once a person expresses interest in providing a sample, the procedures will be the same as those for in-home collections for saliva or blood except that the sample(s) will be collected immediately after obtaining consent rather than mailing the kit to the participant’s home. The collections will be in a private area with dividers separating the areas. Partners will announce the event using the email in Appendix B-5.
Postmortem Biorepository Component
Selecting prospective participants
Eligibility. Once the person has been contacted and has expressed interested in participating in the postmortem collection, the potential participant must be deemed medically eligible based on information from his/her treating neurologist.
Identifying prospective participants. To achieve the target sample size of 40 participants, we estimate that it will be necessary to contact approximately 100.
Demographic composition of biorepository population. The size is too small to recruit a sample that is representative by geographic area, race or ethnicity.
Enrolling participants
Introducing the biorepository. Prospective participants can elect to participate in both components of the biorepository (i.e., in-home biospecimen or postmortem), in only one component, or in neither. Enrollment and specimen collection for all who choose to participate in the in-home component will proceed as outlined in the previous section (In-home Component). Recruitment for the Postmortem Component is described in the next section. For those who elect to participate in both components of the biorepository, the recruitment call will cover both portions. However, calls to obtain consent will be done separately.
Recruitment. A sample of persons indicating an interest in the biorepository will be mailed a packet of information that includes an introductory letter (Appendix C-1a), factsheet (Appendix C-2a), HIPAA authorization form (Appendix C-2b) and consent forms (Appendix C-3 and Appendix C-3a). Approximately one week (5-10 days) after the packet is mailed, McKing biorepository staff will contact the potential participant to determine if he/she has received the packet, answer any questions, and if interested in participating, go over the process. Biorepository staff will ask if the person is interested in participating that he/she sign and return the HIPAA authorization form allowing us to speak to his/her treating neurologist in the self-addressed stamped envelope. Once the HIPAA authorization form is received, we will contact their treating neurologist to confirm eligibility.
Screening prospective participants. McKing biorepository staff will answer all questions about the biorepository and maintain all direct contact with participants except for collecting the specimens. Biorepository staff will consult with the prospective participant’s treating neurologist to ensure that they meet the following criteria: 1) has an expected lifespan of 12-24 months from last date of contact with the treating neurologist; 2) has not been diagnosed with dementia; 3) has not been diagnosed with other neurological diseases; 4) has no plans to go onto a respirator as part of their ALS treatment plan; and 5) has no other medical or family issues that the treating neurologist believes makes the prospective participant a poor candidate for brain donation (Appendix C-2c). Following consultation with the treating neurologist, the biorepository staff will contact the prospective participant to notify them that they meet or do not meet the eligibility criteria for participation (Appendix C-1e and Appendix C-1f).
Informed consent. Within two weeks of confirming a prospective participant’s eligibility to participate in the Postmortem Component, biorepository staff will contact the prospective participant and arrange a time for a call with them and their family member (Appendix C-1f). One week prior to the meeting, a letter to confirm the appointment will be mailed to the participant (Appendix C-1g). At this meeting, biorepository staff will explain the purpose of the biorepository, the process by which the postmortem tissues will be harvested, answer all questions that the prospective participant or family member may have, and review the consent form in detail. If the prospective participant is ready to consent to the biorepository at this meeting, biorepository staff will ask the prospective participant to sign the consent form. The Family Authorization Form (Appendix C-2d) for postmortem donation will be signed by the family member at the same time. The signed consent form and Family Authorization form will be returned to McKing in a self-addressed stamped envelope.
If the prospective participant is not ready to consent to the biorepository during this call, biorepository staff will follow up with them within two weeks following the call to determine if they are interested in participating (Appendix C-1j). If at any time during this process the prospective participant informs biorepository staff that he/she does not want to participate, his/her name will be removed from the biorepository contact list and they will not be contacted again. If after follow-up the prospective participant is not ready to consent, he/she will be considered a passive refusal and will not be contacted again. However, if a prospective participant later contacts staff to say that he/she is ready to consent; he/she may be included if the sample size has not been reached.
McKing will assign a Biorepository ID to each participant at the time his/her consent form has been signed. At this time a letter will be mailed to the participant to thank them for agreeing to participate in the biorepository (Appendix C-1k).
Preparing for specimen collection
Planning for Tissue Recovery. Specimen collection will be coordinated by the National Disease Research Interchange (NDRI), a non-profit, federally funded organization dedicated to retrieval of human tissues and organs for research, prepared, preserved, and shipped according to specific scientific protocols. After an individual is consented, he/she will be given the name of an NDRI staff member that will contact them to answer any questions and help facilitate the donation. McKing biorepository staff and NDRI will develop an individualized tissue recovery plan for each participant within 60 days from their consent to participate. The tissue collection plan used by McKing will include: 1) family primary point of contact, including name and contact information; 2) information on funeral plans, including name and contact information for funeral home; 3) name and contact information for transportation of the participant’s body to and from harvest site; 4) name and contact information for the diener who will harvest the tissues; 5) tissue collection site and contact information; and 6) name and contact information for backup diener in case the primary diener is unavailable at the time of the participant’s death. Once the tissue collection plan is complete, biorepository staff will notify the participant. The family will be provided with a toll-free, 24/7 phone number to call upon death of the participant. McKing will complete a donation plan that will be shared with the participant to make sure his/her family knows what will be done so donation goes smoothly (Appendix C-1l). NDRI is responsible for ensuring all applicable requirements (including consent if required) of the jurisdiction are met before harvesting tissues.
Tracking participants. Biorepository staff will maintain contact with and track all enrolled participants in order to monitor the progression of their disease (Appendix C-1m). Monitoring the participant’s condition will allow biorepository staff to be aware when death is imminent and better prepared to implement the tissue recovery plan. Once every three months, biorepository staff will contact the participant or his/her family via phone to check in. As the participant’s disease progresses and they appear closer to death, biorepository staff will increase their contacts with the participant or their family to once a month. Biorepository staff will document key points of these conversations and file them in the participant’s file.
Family Authorization. At least one family member at the request of the participant will be included in discussions with the participant so that he/she can participate in the decision of the person with ALS to participate in the postmortem specimen collection. The family member will be kept informed of and included in any subsequent discussions with the person with ALS and will be present when the person consents. At the time of death, the family member will contact biorepository staff to arrange transit of the remains for specimen collection. If for any reason the family member decides not to proceed with the donation, the collection will be cancelled.
Specimen collection kits. Individual postmortem tissue collection kits will be assembled by NDRI and distributed to sites to assist in the proper collection and transport of tissue. The kits will include pre-labeled collection supplies such as pre-filled formalin containers, collection cups, and collection forms. Each kit will include an insulated shipper; all required shipping supplies/labels to meet IATA regulations; a pre-paid return FedEx air bill; notification form and packing/shipping instructions. All specimens will be labeled with the Biorepository ID number with an extension for the type of specimen collected. Therefore, Biorepository ID numbers will be the same if a participant agrees to be in both the in-home collection and the postmortem portions of the study.
Collecting specimens
The body will be transported to a site designated for specimen collection. Specimen collection will be coordinated by NDRI. Detailed information on specimen collection can be found in Appendix C-4. The goal of the protocol is to complete all collections and return the body to the funeral home within two days. However, we will allow three days if obstacles are encountered. We will calculate the time from death notification to receipt of the body for each participant.
Brain, spinal cord, and CSF. A contracted diener will obtain the brain, spinal cord, and CSF specimens using standard procedures, which usually take two to three hours. The diener will pack the specimens on ice and a courier will deliver them to the nearest airport for shipping to the Boston University Alzheimer's Disease Center Brain Bank.
Muscle and bone. The diener will obtain muscle and bone specimens during the brain and spinal cord recovery process. Muscle and bone specimens will be deposited in separate formalin-filled vials and a courier will deliver them to for shipping to Fisher BioServices.
Skin. The diener will obtain a skin specimen during the brain and spinal cord recovery process. The skin specimen will be deposited into a separate vial and shipped to Zen-Bio Incorporated for creation of a cell line.
After specimens are collected
Specimens will be tracked from shipment of the specimen collection kit to receipt of specimens. Once we are notified that the specimens have been collected, a letter will be sent to the family of the participant to recognize the donation and to thank them for their support (Appendix C-1n).
Shipping specimens to the laboratory. Brain, spinal cord, and CSF will be shipped to the Boston University Alzheimer's Disease Center Brain Bank. Muscle and bone specimens will be shipped to Fisher BioServices and skin specimen shipped to Zen-Bio.
Laboratory processing. Brain, spinal cord, and CSF specimens will be processed at the Boston University Alzheimer's Disease Center Brain Bank according to procedures detailed in Appendix C-5 and Appendix C-6. Muscle and bone specimens will be storage-ready and will not require additional processing. Once inventoried, samples will be immediately placed into permanent locations and stored at the appropriate temperature for the sample type. The storage unit(s) will be monitored 24/7 by an electronic monitoring system in accordance with pre-approved temperature specifications. The skin specimen will go to ZenBio for cell-line creation.
Unsuccessful recovery. Timing of postmortem tissue collection is critically important. The tissues must be harvested within 48 hours of death and then processed for storage within 24 hours of harvesting. If tissue recovery fails due to family member refusing participation after death or timing problems during the process, these issues will be documented. If the collection fails due to any issue other than family withdrawal, the family will be notified. No follow-up action with the participant’s family member will occur if the family member refuses participation.
Human Subjects Considerations
In-home Component
Biorepository staff will schedule telephone calls with each person interested in participating in the biorepository to go over the consent form (Appendix B-3) and answer questions. At the end of the call, the biorepository staff will ask those interested in participating to sign and date the consent form. All participants will be asked to provide consent themselves, however some persons with ALS have disease progression limiting their physical abilities. Because disease progression varies by individual, it is impossible to predict who, or the number of individuals that might be unable to sign. For individuals unable to sign because of physical disabilities, we are requesting a waiver of documentation. This research is no more than minimal risk and does not include any procedures that would require documentation of consent outside of a research setting. In these cases, we will require that there be a witness to the consenting process and consent of the person. The witness will be requested to sign the consent form. Because this is only a witness and not a legally authorized representative, we would permit the witness to be a family member, caregiver, or friend. The participant will send the signed consent form to McKing in a self-addressed stamped envelope. A copy of the signed consent form will be sent to the participant with his/her thank you letter in the event he/she has any questions. We are requesting permission to not include information addressing GINA on the consent form. Unlike many conditions, it is unlikely that individuals will be unaware that an individual or family is affected by ALS. This information is just as likely, if not more so, to cause discrimination than information about a gene that is only associated with ALS. In addition, it is likely to cause confusion and concern that is unnecessary.
Postmortem Component
Consent to determine eligibility will be obtained through the use of a script and written information sheet and will be documented via the signed HIPAA authorization form. After expressing interest in the postmortem component, the potential participant will be mailed a HIPAA authorization form (Appendix C-2b) to allow biorepository personnel to speak with his/her treating neurologist to determine eligibility (Appendix C-2c). After determining that a person is eligible, biorepository staff will contact the person to schedule a conference call with the potential participant and the family member most likely to be in charge of his/her final arrangements. The biorepository staff will go over the consent form (Appendix C-3) and answer any questions. All participants will be asked to provide consent themselves. However some persons with ALS have disease progression limiting their physical abilities, therefore we are requesting a waiver of documentation for those who are unable to sign. In these cases, we will require that there be a witness to the consenting process and consent of the person. The witness will be requested to sign the consent form. Because this is only a witness and not a legally authorized representative, we would permit the witness to be a family member, caregiver, or friend . In addition, we will ask the family member to sign the Family Authorization Form (Appendix C-2d). This form attests to the family member’s understanding of his/her family member’s wishes regarding postmortem donation and his/her agreement to abide by his/her wishes. Although this form is nonbinding and we would not collect specimens against a family member’s wishes, it involves the family member in the process and makes it more likely he/she will honor the family member’s wishes. A copy of the signed consent form and HIPAA authorization form will be mailed to the participant. We are requesting permission to not include information addressing GINA on the consent form. Unlike many conditions, it is unlikely that individuals will be unaware that an individual or family is affected by ALS. This information is just as likely, if not more so, to cause discrimination than information about a gene that is only associated with ALS. In addition, it is likely to cause confusion and concern that is unnecessary.
Distribution of Specimens
The goal of the National ALS Biorepository is to have specimens available for ALS research. ATSDR will develop an application and review process for researchers to request specimens from the Biorepository. Once the request is approved, Biorepository staff will work with the research to send the approved specimens. Specimens will have the Biorepository ID number on the vials so that Biorepository staff can address questions about specific specimens, however at no time will researcher outside of ATSDR have access to the key. Any epidemiologic data that accompanies the specimens will be coded in such a way as to make inadvertent identification unlikely. For example, exact age will not be provided but will be recoded to age group, e.g., 55 will become 50-59. The Biorepository ID numbers will be added to the National ALS Registry to facilitate the linking of the biospecimens and the epidemiological data.
Specimen Analysis Conducted by the National ALS Registry
Some specimen analyses will be done by the National ALS Registry including those that contribute to one of the purposes outlined in the legislation, i.e., examine appropriate factors, such as environmental and occupational, that might be associated with the disease. Analytic results such as levels of metals in blood will become part of the Registry and available to other researchers. This increases the usefulness of the Registry and Biorepository and decreases duplication of effort.
Metals Analysis
Given that familial ALS occurs in 5%–10% of cases, it has been hypothesized that exposure to environmental factors may interact with genetic susceptibility in the development of ALS. Familial ALS can be caused by mutations in several genes, including C9orf72, SOD1, TDP43, and FUS genes, and these genes also contribute to the development of sporadic ALS (Ajourd-Driss 2015). Proposed environmental risk factors for ALS include exposure to cyanobacteria, various metals (lead, methyl mercury, selenium, zinc, copper and cadmium), pesticides, cigarette smoking, electromagnetic fields (EMF), and electrical shock (Trojsi 2013). The National ALS Registry conducted a pilot biorepository with voluntary participation from registrants. The Registry is unique because it includes persons with ALS (PALS) in the general population while most other human studies have been conducted on occupational cohorts or clinic patients. So far, limited studies have measured environmental chemicals in ALS biospecimens. We will start our exploratory analyses of environmental chemicals for an association with ALS. We will first focus on metals and metalloids, including mercury, cadmium, lead, manganese, arsenic, chromium, selenium, copper, and possibly others (that are tested in conjunction with them, such as beryllium and uranium measured in the lead assay). To examine the hypothesis that exposure to metals is associated with the development of ALS, we will use biospecimens that were collected as part of the ALS biorepository. We anticipate the sample size will be between 300 and 600, depending on the registrants’ response. The NCEH Division of Laboratory Sciences will conduct the bioassays using each aliquot tube labeled only with the participant’s coded ID number. No personally identifiable information will be shared. Because the pilot biorepository did not include a control group, we will utilize the NHANES data to obtain estimates of the demographic distribution of metal levels for the US Population and test for:
• Null hypothesis: ALS registrants’ distribution of metal levels is the same as a similar group of the US Population
• Alternative hypothesis: ALS registrants have higher metal levels than a similar group of the US Population
We will fit multivariate Cox proportional hazards models to determine whether survival time is associated with metal levels. The dependent variable will be interval from registration to death. The date of death and multiple causes of death will be obtained from the National Death Index. We will model the metal levels as continuous variables after making proper transformations for each metal. All models will include the following covariates: sex, age at diagnosis, race/ethnicity, and smoking status. We will also examine the available exposure information by linking the ALS registrants’ survey data.
Genomic Analysis
Finally, the DNA samples of registrants will be genotyped using the NeuroX version 2 SNP chip (from Illumina) at Dr. Bryan Traynor’s laboratory at the NIH. This SNP chip consists of a standard component with additional custom content. The Illumina HumanOnniCore forms the backbone of this array, assaying about 300,000 common SNPs across the human genome. The custom content focuses on neurodegeneration, and assays about 100k SNPs. These custom markers target specific regions of the genome implicated in neurological diseases and mutations in genes that cause ALS, Parkinson's disease, Alzheimer's disease, multiple system atrophy and other neurological diseases (Nalls 2015). Dr. Bryan Traynor has an IRB exemption to ascertain individuals with a clinical diagnosis of a movement disorder, their affected and unaffected family members, and unrelated, healthy individuals (to provide control samples); to characterize their phenotypes; and to identify and further characterize genetic contributions to etiology by collecting blood samples, saliva samples, and/or skin biopsies on these individuals for DNA and induced Pluripotent stem cell line preparation. He will be receiving deidentified DNA samples from the ALS biorepository for his testing. Genetic test results on ALS registrants will be transmitted to ATSDR to link with the ALS registrant, their survey data and other testing that is done by the biorepository. We will examine the potential relationships between the metals shown to be associated with ALS and mutations in known neurodegenerative genes (C9orf72, SOD1, TDP43, and FUS genes and others). We will assemble appropriate control groups for future metals and genetic test comparisons.
Βeta-N-methylamino-L-alanine (BMAA) Analysis
Matched brain, spinal cord, and cerebral spinal fluid (CSF) material from 25 registrants will be analyzed to detect and quantify BMAA. Members of the Emergency Response Branch (ERB) and Tobacco and Volatiles Branch (TVB) of the Division of Laboratory Sciences (DLS), National Center for Environmental Health (NCEH) will support this investigation via the development and validation of methods to detect and quantify BMAA in human tissues. Biorepository samples will be delivered to the Incident Response Laboratory (IRL), where IRL analysts will ensure samples are cataloged, blinded and disseminated to analysts. Sample preparation of brain tissue, spinal cord and CSF will extract the small molecules from the matrices and coupled liquid chromatography (LC) with mass spectrometry (MS) analysis will provide the concentration data. Brain and spinal cord tissue samples will be prepared using bead pulverization, followed by an organic extraction; CSF will have the proteins removed by precipitation. All organics will be hydrolyzed with strong acid and processed by solid-phase extraction (SPE). The LC/MS analysis of BMAA and its isomers will be done by a method previously published by TVB that utilizes an ion pairing technique for baseline separations of constitutional isomers; the LC/MS analysis will include isomer compounds of BMAA prevalent in algae to ensure there are no false positives. Analysts from TVB will upload the results into STARLIMS and the IRL will provide EHSB with an interpretation key. We will assemble appropriate control samples for future BMAA comparisons in persons with ALS and people with no ALS diagnosis.
Results of Research
Results of research will not be returned to participants for several reasons. It is unclear when specific samples will be used for research and could be many years in the future when the person with ALS is no longer alive. The National ALS Registry and the National ALS Biorepository do not collect information on next-of-kin. In addition, the Registry and the Biorepository many not actually have the results of research conducted by approved researchers outside of CDC/ATSDR. Therefore, it is not logistically possible to provide the results of research to participants or their families. ATSDR will post aggregate results of research on their website for the ALS community.
References
Ahmed A, Wicklund MP. Amyotrophic lateral sclerosis: what role does environment play? Neurol Clin. 2011 Aug;29(3):689-711.
Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim Biophys Acta. 2015. 1852(4):679-684.
Allen KD, Kasarskis EJ, Bedlack RS, Rozear MP, Morgenlander JC, Sabet A, Sams L, Lindquist JH, Harrelson
M L, Coffman C J, Oddone EZ. The National Registry of Veterans with amyotrophic lateral sclerosis
The National Registry of Veterans with amyotrophic lateral sclerosis. Neuroepidemiology 2008 30(3):180-90.
Alonso A, Logroscino G, Hernán MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2010 Nov;81(11):1249-52.
Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009 Nov 17;73(20):1693-8.
Bowser R, Turner MR, Shefner J. Biomarkers in amyotrophic lateral sclerosis: opportunities and limitations. Nat Rev Neurol. 2011 Oct 11;7(11):631-8.
Chiò A, Schymick JC, Restagno G, et al. A two-stage genome-wide association study of sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2009 Apr 15;18(8):1524-32.
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011 Oct 20;72(2):245-56.
Goullé JP, Saussereau E, Mahieu L, et al. Application of inductively coupled plasma mass spectrometry multielement analysis in fingernail and toenail as a biomarker of metal exposure. J Anal Toxicol. 2009 Mar;33(2):92-8.
Gwinn K, Corriveau RA, Mitsumoto H, Bednarz K, Brown RH Jr, Cudkowicz M, Gordon PH, Hardy J, Kasarskis EJ, Kaufmann P, Miller R, Sorenson E, Tandan R, Traynor BJ, Nash J, Sherman A, Mailman MD, Ostell J, Bruijn L, Cwik V, Rich SS, Singleton A, Refolo L,. Andrews J, Zhang R, Conwit R, Keller MA; ALS Research Group. Amyotrophic lateral sclerosis: an emerging era of collaborative gene discovery. PLoS One. 2007 Dec 5;2(12):e1254
Fang F, Kamel F, Lichtenstein P, et al. Familial aggregation of amyotrophic lateral sclerosis. Ann Neurol. 2009 Jul;66(1):94-9.
International Society for Biological and Environmental Repositories (ISBER). Best practices for repositories: collection, storage, retrieval, and distribution of biological materials for research. 3rd ed. Available at: https://c.ymcdn.com/sites/www.isber.org/resource/resmgr/Files/ISBER_Best_Practices_3rd_Edi.pdf
Kasarskis EJ, Dominic K, Oddone E. The National Registry of Veterans with Amyotrophic Lateral Sclerosis: Department of Veterans Affairs Cooperative Studies Program (SCSP) #500a. Amyotroph Lateral Scler Other Motor Neuron Disord 2004 5(Suppl1):129-32.
Lill CM, Abel O, Bertram L, Al-Chalabi A. Keeping up with genetic discoveries in amyotrophic lateral sclerosis: the ALSoD and ALSGene databases. Amyotroph Lateral Scler. 2011 Jul;12(4):238-49.
Millecamps S, Da Barroca S, Cazeneuve C, Salachas F, Pradat PF, Danel-Brunaud V, Vandenberghe N, Lacomblez L, Le Forestier N, Bruneteau G, Camu W, Brice A, Meininger V, LeGuern E. Questioning on the role of D amino acid oxidase in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):E107
Nalls MA., Jose B, Dena GH, Margaux FK,, Elisa M, Alan ER et al. NeuroX, a fast and efficient genotyping platform for investigation of neurodegenerative diseases. Neurobiol Aging. 2015. 36(3):1605.e7-12.
National Biospecimen Network Blueprint, Andrew Friede, Ruth Grossman, Rachel Hunt, Rose Maria Li, and Susan Stern, eds. Constella Group, Inc., Durham, NC, 2003. Available at: http://biospecimens.cancer.gov/global/pdfs/FINAL_NBN_Blueprint.pdf
Otto M, Bowser R, Turner M, et al. Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph Lateral Scler. 2012 Jan;13(1):1-10.
Public Population Project in Genomics (P3G) Observatory. Repository of information and tools. Sample collection and processing. Available at: http://www.p3gobservatory.org/repository/sampleCollection.htm
Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011 Oct 20;72(2):257-68.
Schmidt S, Allen KD, Loiacono VT, et al. Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis: the GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30(3):191-204.
Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010 Apr 15;291(1-2):22-9.
Sherman A, Bowser R, Grasso D, et al. Proposed BioRepository platform solution for the ALS research community. Amyotroph Lateral Scler. 2011 Jan;12(1):11-6.
Siddique T, Ajroud-Driss G. Familial amyotrophic lateral sclerosis, a historical perspective. Acta Myol 2011 Oct;30(2):117-20.
Storandt M, Head D, Fagan AM, Holtzman DM, Morris JC. Toward a multifactorial model of Alzheimer disease. Neurobiol Aging. 2012 Jan 17.
Trojsi F, Monsurrò MR, and TedeschG. Exposure to Environmental Toxicants and Pathogenesis of Amyotrophic Lateral Sclerosis: State of the Art and Research Perspectives. Int J Mol Sci. 2013. 14(8): 15286–15311.
Wu CH, Fallini C, Ticozzi N, et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature. 2012 Jul 15. doi: 10.1038/nature11280. [Epub ahead of print]
Table 1. Factors previously studied for association with ALS, with selected examples citing relevant biomarkers or exposures.
Factor/example |
Review or study |
Biomarker or history of exposure |
Infectious agents |
||
EX: Enterovirus |
Vandenberghe 2010. PMID 19900148 |
PCR for enterovirus RNA (CSF, fixed or frozen brain or spinal cord) |
Cyanobacteria toxins |
Cox 2009. PMID 19254284 |
HPLC/mass spectrometry analysis of protein-bound BMAA (archived brain or spinal cord) |
Pesticides |
||
EX: Organophosphates |
Johnson 2009. PMID 19632272 |
Paraoxonase enzyme activity, PON1 genotype (blood) |
Metals |
||
EX: Lead, mercury |
Johnson 2009. PMID 19632272 |
Metals and organometallic compounds (brain, blood, CSF) |
Drugs and other chemicals |
||
EX: Statins |
Sorensen 2009. PMID 19930099 |
Medical history |
Formaldehyde |
Weisskopf 2009. PMC2765376 |
Occupational history |
Injury |
||
EX: Head trauma |
Schmidt 2011. PMC2840700 |
A-beta protein deposition (brain), interaction with APOE genotype (blood) |
Electrical shock |
Abhinav 2007. PMC2117843 |
History of lightning strike or other electrical injury |
Occupation |
||
EX: Military service |
Weisskopf 2005. PMID 15642900 |
Occupational history |
Professional soccer |
Chio 2009. PMID 19267274 |
Occupational history |
Smoking
|
Alonso 2010. PMID 20639382 |
Smoking history |
Diet |
Morozova 2008. PMID 18300717 |
Dietary history |
Table 2. Frequencies of mutations in five genes associated with familial ALS in a study of 162 families (Millecamps 2010).
-
Gene
Families
(n)
Distinct mutations
(n)
Novel mutations
(n)
SOD1
20
18
3
TARDBP
7
6
2
FUS
7
5
2
ANG
1
1
0
VAPB
1
1
0
Table 3. Pathophysiologic processes with hypothesized roles in ALS, with selected examples citing approaches to measurement.
Pathophysiologic process |
Review or study |
Examples of measurement approaches |
Gene expression and RNA metabolism |
||
EX: Gene expression |
Mougeot 2011. PMC3219589 |
Microarray (peripheral blood lymphocytes) |
EX: RNA processing |
Lagier-Tourenne 2010. PMC3167692 |
Immunohistochemistry (brain and spinal cord) |
EX: Endogenous retrovirus |
Douville 2011. PMC3052883 |
PCR, sequencing, immunohistochemistry (brain) |
Protein expression and metabolism |
||
EX: Protein expression |
Kudo 2010. 20530642 |
Laser capture microdissection, tissue microarray (spinal cord) |
Protein degradation |
Ryberg 2010. PMID 20583124 |
Mass spectrometry-proteomics (cerebrospinal fluid) |
Protein misfolding |
Bosco 2011. PMC2967729 |
Immunoblot, immunohistochemistry (spinal cord) |
Energy metabolism |
||
EX: Lipid metabolism |
Dupuis 2011. PMID 21035400 |
Lipid levels (blood), mitochondrial function (muscle) |
Oxidative stress |
Lawton 2012. PMID 22117131 |
Mass spectrometry-metabolomics (plasma) |
Inflammation |
||
EX: Cytokines |
Fiala 2010. PMC2992053 |
ELISA (serum), microarray, immunohistochemistry (spinal cord) |
Astrocyte toxicity |
Haidet-Phillips 2011. PMID 21832997 |
Cell co-culture, mircroarray (spinal cord) |
Table 4. Proposed desirable minimum clinical dataset for ALS biomarker studies (Otto 2012).
Category |
Clinical information |
Generic |
Age |
Gender |
|
Ethnicity |
|
Drugs |
|
Past medical history |
|
Family history of neurodegenerative disorders (back two generations) |
|
Phenotype |
Diagnostic certainty (ALS by revised El Escorial criteria plus PLS or PMA), confirmed each time-point. |
Symptom onset (months/years) |
|
Date of diagnosis |
|
Site of onset (bulbar, upper/lower limb, respiratory) |
|
Regional phenotypes (flail arm, flail leg, progressive bulbar palsy, upper versus lower motor neuron-predominant ALS, cognitive involvement e.g. ALS-FTD) |
|
Evaluation |
ALSFRS-R |
Forced Vital Capacity |
|
Upper/lower motor neuron involvement |
|
Body Mass Index |
|
Cognitive assessment, e.g. Verbal Fluency Index |
|
Interventions |
Gastrostomy |
Non-invasive ventilation |
|
Cough-assist device |
|
Tracheostomy |
|
Date of death (with consideration of stratification of patients according to time of sampling in relation to overall disease course) |
Copy of Table IV in Otto 2012.
Table 5. Biorepositories and brain banks with samples from people with ALS
Biorepository |
Sponsor |
Home |
Sample types |
Number with ALS |
Access |
Clinical biorepositories |
|||||
Northeast ALS Consortium (NEALS) |
Consortium |
Massachusetts General Hospital Boston, MA |
serum, plasma, CSF, whole blood, extracted DNA, urine |
5 clinical trials and 7 biomarker studies, each enrolling ~30-300 participants; ongoing open enrollment |
applications reviewed by NEALS committee, with priority given to NEALS and other sites that contributed samples |
NINDS Motor Neuron Disease Collection
|
National Institute for Neurologic Diseases and Stroke (NINDS, NIH) |
Coriell Institute for Medical Research Camden, NJ (USA) |
DNA, fibroblasts, immortalized lymphocytes |
2021 persons |
available on order with material transfer agreement and statement of research intent |
Population-based biorepository |
|||||
National Registry of Veterans with ALS |
Veterans Administration (VA) |
Boston VA Medical Center Boston, MA |
DNA (blood 85%, saliva 15%) |
>1200 persons |
collaborative research with Duke University |
Brain banks |
|||||
VA Biorepository (VAB) Brain Bank |
Veterans Administration (VA) |
Southern Arizona VA Healthcare System Tucson, AZ |
brain, spinal cord |
not specified |
not specified |
MRC London Brain Bank for Neurodegenerative Diseases |
Medical Research Council (MRC) London, UK |
Institute of Psychiatry, King’s College London, UK |
fixed and frozen human brain tissue and spinal cord, sections on slides, frozen CSF, micro-dissected regions, extracted DNA/RNA |
189 persons with motor neuron disease |
available to researchers by application |
Table 6. UK Biobank specimen collection protocol (adapted from Elliott 2008).
Collection priority |
Sample preservative |
Volume (ml) |
Transport temp (oC) |
Specifications |
1 |
EDTA |
9 |
4 |
Spray dried to give a final concentration of 1.8 mg/ml blood |
2 |
Lithium heparin |
8 |
4 |
*Plasma separation tube; spray dried to give a final concentration of 17 IU/ml. |
3 |
Clot activator |
8 |
4 |
*Serum separation tube; silica clot accelerator used in plastic collection tubes. |
4 |
EDTA |
9 |
4 |
|
5 |
Acid citrate dextrose |
6 |
18 |
Tube contains 1.0 ml of solution giving 2.2 mg/ml blood sodium citrate, 0.8 mg/ml citric acid, 2.45 mg/ml dextrose |
6 |
EDTA |
4 |
4 |
|
|
|
|
|
*indicates use of gel plug that forms a barrier to cellular material but allows serum or plasma to pass through |
|
Urine |
9 |
4 |
|
Table 7. Relative advantages of different specimen types for biomarker research (Otto 2012).
Characteristic |
Blood* |
CSF |
Urine |
Saliva |
Skin |
Muscle |
Proximity to CNS pathology |
++ |
+++ |
+ |
+ |
+ |
+ |
Less molecular complexity |
+ |
+ |
++ |
+++ |
++ |
++ |
Less invasive |
++ |
+ |
+++ |
+++ |
+ |
+ |
Practicality of sampling |
+++ |
++ |
+++ |
++ |
+ |
+ |
Ease of handling for storage |
++ |
+ |
++ |
+ |
+ |
+ |
Resistance to exogenous drug contamination |
+ |
+++ |
+ |
++ |
++ |
++ |
Candidate molecules to date |
++ |
+++ |
+ |
+ |
+ |
+ |
Potential for DNA/RNA analysis |
+++ |
+ |
+ |
++ |
+++ |
+++ |
+++ high; ++ moderate; + weak |
||||||
*Plasma versus serum needs to be specified; serum may have advantages for the stability of some proteins, e.g. immunoglobulins. EDTA sample will be needed for DNA or RNA studies. |
||||||
Adapted from Table V in Otto 2012. Note that skin specimens will not be collected for the National ALS Biorepository.
Table 8. Proposed minimum specimen collection protocol, with examples of potential analyses using each sample type.
Collection priority |
Sample preservative |
# tubes |
ml / tube |
Fractions |
Examples |
Blood |
|||||
1 |
K2EDTA |
1 |
10 |
White cells (buffy coat), red cells, plasma |
DNA, proteins, red blood cell lipids |
2 |
K2EDTA |
1 |
6 |
Whole blood |
Lead, other metals |
3 |
Plain, (no anticoagulant |
1 |
10 |
Serum |
Clinical biochemistries, metabolic products, other small molecules |
4 |
CPT |
1 |
8.0 |
Cells |
Cell line creation |
5 |
PAXgene RNA |
1-2 |
5 |
RNA-stabilized whole blood |
Intracellular RNA |
|
|||||
Urine
|
9 |
-- |
Electrolytes, environmental chemicals, metabolic products |
||
Nail clippings |
-- |
-- |
Metals |
||
Hair clippings |
-- |
-- |
Metals |
||
Saliva1 (Oragene Collection Kit) |
2 |
-- |
DNA |
||
Sources: Eliott 2008, Holland 2003, Otto 2012
1Note: saliva samples will not be collected by technicians; self-collection of saliva will be used only when blood collection fails.
Drawing the 6.0-mL EDTA in second order allows the butterfly needle line to be flushed of potential heavy metal contamination beforehand. The PAXgene tubes are always collected last.
When blood is collected from a participant, the Vacutainers™ are inverted 10 times to mix the anti-coagulant/preservative with the whole blood (or to activate clotting in the red-top tubes with no anticoagulant). Using the butterfly collection device allows the phlebotomist to mix the previous tube thoroughly while the next tube is being collected.
Figure 1. Genetic association studies of sporadic ALS by year, 2001-2011 (as of January 2012).
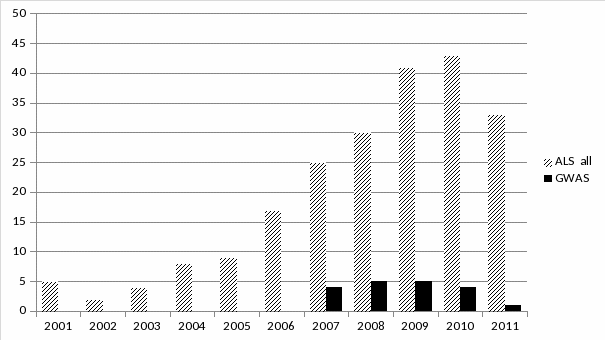
GWAS = genome-wide association studies
Data from HuGE Navigator, www.hugenavigator.net.
Appendix A. Request for additional information
Research is an important part of the National ALS Registry. Specimens are needed for much of this research. The National ALS Biorepository is a new part of the National ALS Registry. If you are interested in learning more about the Biorepository, please check the “I Agree” box. A sample of interested PALS will get information packets.
I Agree
If “I Agree”, new screen
I would like to learn more about donating biospecimens to the National ALS Biorepository.
I would like to learn more about donating postmortem tissues to the National ALS Biorepository.
I would like to learn more about donating both biospecimens and postmortem tissues to the National ALS Biorepository
Please provide your mailing address and phone number. This information will be used to make sure we ask PALS from all over the US to take part. We will also use this information to contact you if you are selected.
Mailing address: __________________________City:_____________ State: _______ Zip code: _______
Phone number: ( ) ____-_______
Appendix B. In-home Biorepository Component
Appendix B-1. Communications


This will be sent to participants who expressed an interest in participating only in the biospecimen portion of the biorepository. A copy of the fact sheet and consent form will be enclosed with this letter.
[Date]
Name
Address
City, State Zip
Dear [Participant Name]:
The Agency for Toxic Substances and Disease Registry (ATSDR) has launched the National ALS Biorepository (Biorepository). The Biorepository is a new part of the National ALS Registry. They have contracted with McKing Consulting (McKing) to manage the Biorepository. McKing would like to thank you for your interest in the Biorepository. The Biorepository will collect, store and distribute specimens from individuals with ALS. You are free to join or not. Your decision will have no impact on your eligibility to take part in the National ALS Registry or any future studies.
We are asking people who are enrolled in the National ALS Registry to donate biological specimens. This part of the Biorepository, may collect blood, urine, hair and nail specimens at your home. If you choose to take part in this part of the Biorepository, you will be asked to answer a few questions and to provide blood, urine, hair and nail specimens.
We are enclosing a fact sheet that explains the biospecimen part of the Biorepository. It also has answers to some frequently asked questions. We have enclosed a copy of the consent form for the Biorepository. Signing this form indicates that you agree to take part in the Biorepository. We will call you to review the consent form and answer any questions. If any part of this form is not clear to you, be sure to ask questions. Do not sign until you get answers to all of your questions. Once you have reviewed the consent form and agreed to participate in the biospecimen part of the Biorepository, we will set up an appointment for a phlebotomist to come to your home and collect specimens.
Your specimens will be saved and banked for future ALS research. We will store your specimens with some data about you, such as your age, race, and sex. We will not put your name on the specimen. We do not know how long we will keep these specimens. All information you provide will be kept private to the extent allowed by law. To protect your privacy your name will not appear with any of your information. A summary of the results of this specimen collection will be posted on the National ALS Registry website. Individuals will not be identified. Taking part in this Biorepository may help us learn more about ALS. Although the Biorepository will not benefit you directly it may be of future benefit to others.
If you have any questions or want more information, please contact the Biorepository Coordinator, Laurie Wagner by email at lwagner@secure.mcking.com or by phone at 1-855-874-6912.
Thank you for supporting the National ALS Biorepository. We look forward to hearing from you soon.
Sincerely,
Wendy E. Kaye, PhD Senior Scientist, National ALS Biorepository
Enclosures
Reading Level 9.9 (changing biorepository with study 7.9)

This will be sent to participants who expressed an interest in participating only in the biospecimen portion of the biorepository. A copy of the fact sheet and consent form will be enclosed with this letter.
[Date]
Name
Address
City, State Zip
Dear [Participant Name]:
The Agency for Toxic Substances and Disease Registry (ATSDR) has launched the National ALS Biorepository (Biorepository). The Biorepository is a new part of the National ALS Registry. They have contracted with McKing Consulting (McKing) to manage the Biorepository. McKing would like to thank you for your interest in the Biorepository. The Biorepository will collect, store and distribute specimens from individuals with ALS. You are free to join or not. Your decision will have no impact on your eligibility to take part in the National ALS Registry or any future studies.
We are asking people who are enrolled in the National ALS Registry to donate biological specimens. This part of the Biorepository, will collect a saliva specimen. If you choose to take part in this part of the Biorepository, you will be asked to provide a saliva specimen using a self-collection kit that will be mailed to your home.
We are enclosing a fact sheet that explains the saliva collection part of the Biorepository. It also has answers to some frequently asked questions. We have enclosed a copy of the consent form for the Biorepository. Signing this form indicates that you agree to take part in the Biorepository. We will call you to review the consent form and answer any questions. If any part of this form is not clear to you, be sure to ask questions. Do not sign until you get answers to all of your questions. Once you have reviewed the consent form and agreed to participate in the biospecimen part of the Biorepository, we will mail a kit to your home to provide a saliva specimen at your convenience.
Your specimens will be saved and banked for future ALS research. We will store your specimens with some data about you, such as your age, race, and sex. We will not put your name on the specimen. We do not know how long we will keep these specimens. All information you provide will be kept private to the extent allowed by law. To protect your privacy your name will not appear with any of your information. A summary of the results of this specimen collection will be posted on the National ALS Registry website. Individuals will not be identified. Taking part in this Biorepository may help us learn more about ALS. Although the Biorepository will not benefit you directly it may be of future benefit to others.
If you have any questions or want more information, please contact the Biorepository Coordinator, Laurie Wagner by email at lwagner@secure.mcking.com or by phone at 1-855-874-6912.
Thank you for supporting the National ALS Biorepository. We look forward to hearing from you soon.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Enclosures
Reading Level 10 (changing biorepository with study 8)
B-1b. – Phone script to schedule appointment, review instructions, and obtain consent
This is a phone script for making calls to individuals who received an information packet. The purpose of this call is to determine if the individual would like to participate and if so, consent the individual through a thorough review of the consent form. After the individual has provided consent, make an appointment for biospecimen collection.
INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with potential participant
IF PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
If person asks on phone for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository
Ask if he/she received the information packet
Inform participant that you are calling to review the consent form
Ask participant if they have had a chance to review the consent form and have a copy available at this time
a) If participant does not have a copy of the consent form available:
Offer to send or email another copy and reschedule a time to call back (depending on their mode of contact.
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
Remind participant to review consent form and to have a copy available for the next call
Thank them and end call
If participant does have a copy of consent form but has not reviewed it:
Allow participant time to review consent form while you wait on the phone
Ask if participant wants to continue to participate in the biorepository
If participant does have a copy available and has reviewed consent form:
Ask if participant wants to continue to participate in the biorepository
IF PARTICIPANT DOES NOT WANT TO CONTINUE TO PARTICIPATE IN THE BIOREPOSITORY:
Remind participant of your name
Thank them and end call
IF PARTICIPANT DOES WANT TO CONTINUE TO PARTICIPATE IN THE BIOREPOSITORY:
Review each section of the consent form
Ask participant if they have any questions at the end of each section
Ask participant if they still want to participate in biorepository
If no,
Remind participant of your name
Thank them and end call
If yes,
Ask participant or witness to sign the consent form
Inform participant that the signed copy should be mailed back in the pre-stamped envelope sent with the information packet
CONFIRM PARTICIPANT CONTACT INFORMATION:
Review the contact information that you have on file and make any corrections necessary; (name, address, phone, email etc.)
Ask if the participant prefers US mail or email communication.
SCHEDULE APPONTMENT DATE AND TIME:
WHAT’S NEXT:
Schedule appointment based on collection method selected by participant.
IF PARTICIPANT SELECTED IN-HOME COLLECTION BY PHLEBOTOMIST
Schedule appointment for in-home specimen collection
Inform participant that a box containing the collection kit should arrive at their home
Inform participant that the phlebotomist will call them 1 day before their appointment as a reminder
Ask participant if there is anyone in the household that you can speak to about the biorepository if they are not available to come to the phone
If no,
Make note in database not to speak with anyone else in the home about this biorepository
If yes,
Document name and relationship to participant
IF PARTICIPANT SELECTED IN-HOME COLLECTION BY HEALTHCARE WORKER
Schedule appointment for in-home collection by healthcare worker
Ask participant about healthcare workers’ schedule and/or availability
Ask for potential dates the draw could occur and when the participant can confirm appointment
Add potential date(s) to scheduling spreadsheet and schedule a follow up call with the participant to confirm draw date/time as needed
Inform participant that a box containing the collection kit should arrive at their home
Ask participant if there is anyone in the household that you can speak to about the biorepository if they are not available to come to the phone
If no,
Make note in database not to speak with anyone else in the home about this biorepository
If yes,
Document name and relationship to participant
IF PARTICIPANT SELECTED DOCTOR’S OFFICE COLLECTION
Ask participant when their next appointment is scheduled.
Ask participant the name of the doctor’s office and doctor contact information.
Inform the participant that a member of the biorepository team will call the doctor’s office to confirm that the collection can take place. Once confirmation is received, the participant will be notified.
Inform participant that a box containing the collection kit should arrive at their home. Remind the participant to take the kit with them to the doctor’s office. Only the supplies in the kit will be used for the collection.
IN CLOSING:
Ask participant if they have any questions
Remind participant of your name
Thank them and end call
B-1c. – Letter/email appointment confirmation and instructions
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent is obtained. This letter will be sent once the collection appointment is confirmed with a phlebotomist. The purpose of the letter is to confirm the appointment and provide instructions to the participant on preparatory activities.
[Date]
Name
Address
City, State, Zip Code
Dear [Participant Name]:
This letter is to confirm your appointment for a phlebotomist to come to your home and collect the following specimens:
Blood
Urine
Hair clippings
Finger nail clippings
Your specimen collection appointment is scheduled for
(Day/Date): _________________ at (Time): __________________.
Below are instructions to follow prior to your appointment:
Do not cut your hair for at least 1 week before your appointment. [Delete if not collecting hair]
Hair should be free of all gels, oils and hair creams or sprays prior to sample collection. The hair to be collected should be untreated (not permed, dyed or bleached). [Delete if not collecting hair]
Do not cut your fingernails for at least 1 week before your appointment. Please remove nail polish for the day of your appointment. [Delete if not collecting fingernails]
You will find a urine collection cup in the specimen kit sent to your house. Please provide your urine specimen the morning before your appointment.
Drink plenty of water the days leading up to your blood draw and on the day of your blood draw.
If you have any questions feel free to contact me by phone at 1-855-874-6912 or by email at lwagner@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Laurie Wagner, MPH
Coordinator, National ALS Biorepository
Enclosure
Reading Level 6.8
B-1c.1 – Letter/email appointment confirmation and instructions for just blood draw
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent is obtained. This letter will be sent once the collection appointment is confirmed with a phlebotomist. The purpose of the letter is to confirm the appointment and provide instructions to the participant on preparatory activities.
[Date]
Name
Address
City, State Zip
Dear [Participant Name]:
This letter is to confirm your appointment for a phlebotomist to come to your home and collect the following specimens:
Blood
Urine
Hair clippings
Finger nail clippings
Your specimen collection appointment is scheduled for
(Day/Date): _________________ at (Time): __________________.
Below are instructions to follow prior to your appointment:
Drink plenty of water the days leading up to your blood draw and on the day of your blood draw.
If you have any questions feel free to contact me by phone at 1-855-874-6912 or by email at lwagner@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Laurie Wagner, MPH
Coordinator
National ALS Biorepository
Enclosure
B-1d. – Phone script to reschedule appointment
This is a phone script for individuals that need to reschedule their appointment for the biospecimen collection. The appointment can be rescheduled up to 2 times after which we will remove participant from active participant list and he/she can contact us when he/she is ready to have specimens collected.
INCOMING CALL INTRODUCTION:
Answer phone, state your name and that you are with the National ALS Biorepository
Ask potential participant how you can help them
OUTGOING CALL INTRODUCTION
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF PARTICIPANT WANTS TO CANCEL THEIR APPOINTMENT:
Look up participant name in database
Verify date and time of appointment
Cancel appointment
Ask if participant would like to reschedule their appointment
IF PARTICIPANT DOES NOT WANT TO RESCHEDULE THEIR APPOINTMENT:
If collection kit has already been shipped to participant, provide instructions on returning the kit
Thank them and end call (remove name from future contact lists)
IF PARTICIPANT DOES WANT TO RESCHEDULE THEIR APPOINTMENT:
Look up participant name in database
Determine how many times they have canceled appointment (if more than 2 times then do not reschedule appointment, let participant call when they want specimens collected then reschedule)
Schedule new appointment
Day/Date: ___________________________________
Time (am/pm):________________________________
CONFIRM PARTICIPANT CONTACT INFORMATION:
Read participant the contact information that you have on file and make any necessary corrections; (name, address, phone, email)
WHAT’S NEXT:
Confirm participant has specimen collection box.
Ask them to place the box in a safe place because it will be used at the rescheduled appointment.
IN CLOSING:
Ask if participant has any questions
Remind participant of your name
Thank them and end call
B-1e. – Email/letter blood draw failure
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent is obtained. This letter will be sent to the participant if the blood draw failed during the in-home collection. The letter offers the participant the opportunity to complete an alternative form of DNA collection through a self-collection saliva kit.
[Date]
Dear [Participant Name]:
Thank you for agreeing to take part in the National ALS Biorepository. We are sorry we were not able to take your blood during our visit. We would like to know if you would like to donate a saliva specimen instead. We have enclosed a kit and directions. If you would like to provide a saliva specimen, please follow the enclosed directions. Please return the saliva specimen using the pre-paid FedEx air bill and original shipping box.
If you have any questions, please contact Laurie Wagner by phone at 1-855-874-6912 or by email at lwagner@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Laurie Wagner, MPH
Coordinator, National ALS Biorepository
Reading level 10.0 (replacing biorepository with study 8.9)
B-1f. – Phone script for failed blood draw or unusable blood
This is a phone script for participants whose blood draws were unusable because the specimen was damaged during transit or processing or the participants was unable to give blood. The purpose of the call is to inform the participant the specimen is unusable as well as offer an alternative collection of DNA through a self-collected saliva kit.
INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with participant
IF PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT.
Day/Date: ___________________________
Time: _______________________________
If person asks for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the PALS know that you are calling on behalf of the National ALS Biorepository
Inform them that we are unable to use their blood because of a lab/shipping error OR that we were unable to obtain blood
Describe option for failed blood draw (saliva only)
Ask if they would like to provide a saliva sample
For those whose blood was unusable because of shipping or laboratory error, a second blood draw will be considered if the person offers. Please consult with the Biorepository Director or Coordinator.
IF PARTICIPANT DOES NOT WANT TO PROVIDE A SALIVA SAMPLE:
Give participant the biorepository phone number
Thank them and end call
IF PARTICIPANT DOES WANT TO PROVIDE A SALIVA SAMPLE:
Thank them for agreeing to provide saliva sample
Let them know that you will mail a saliva self-collection kit
IF PARTICIPANT VOLUNTEERS TO HAVE BLOOD SAMPLE DRAWN AGAIN (DO NOT ASK THEM) SCHEDULE A NEW APPOINTMENT:
Day/Date: ___________________________
Time: _______________________________
CONFIRM PARTICIPANT CONTACT INFORMATION:
Review the contact information that you have on file and make any corrections necessary; (name, address, phone, email)
WHAT’S NEXT:
Saliva sample:
Let participant know that the saliva kit should arrive within the next week with instructions.
Blood sample:
Let participant know that only blood will be drawn and no other specimens collected.
Confirm appointment, blood draw must be at least 8 weeks since the first draw.
IN CLOSING:
Ask if participant has any questions
Remind participant of your name
Thank them and end call
B-1g. – Email/letter for saliva collection
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent if the blood sample was damaged during transit or processing and becomes unusable. The letter offers the participant the opportunity to complete an alternative form of DNA collection through a self-collection saliva kit.
[Date]
Dear [Participant Name]:
Thank you for taking part in the National ALS Biorepository. We are sorry to inform you that we are unable to use your blood because of a lab/shipping error. We would like to know if you would like to donate a saliva specimen instead. We have enclosed a saliva specimen collection kit and directions. If you would like to provide a saliva specimen, please follow the enclosed directions
If you have any questions, please contact Laurie Wagner by phone at 1-855-874-6912 or by email at lwagner@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Laurie Wagner, MPH
Coordinator, National ALS Biorepository
Reading level 10.6 (without study name 9.4)
B-1h. – Phone script follow-up saliva collection
This is a phone script for following up on saliva kits that have not been returned. The purpose of this call is to confirm the saliva kit has arrived at the participant’s home, remind them to complete the collection and return it to the laboratory.
INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with potential participant
IF PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT:
Day/Date: _____________________
Time: ________________________
If person asks for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository
Ask if they received the saliva collection kit
IF PARTICIPANT DID NOT RECEIVE THE SALIVA SAMPLE COLLECTION KIT:
Let participant know that you will mail another saliva collection kit
CONFIRM PARTICIPANT CONTACT INFORMATION:
Review the contact information that you have on file and make any corrections necessary; (name, address, phone, email)
WHAT’S NEXT:
Let participant know that the saliva kit should arrive within 1 week with instructions and to call if they have any questions once it arrives
Ask participant to mail kit back within 1 week after they provide sample
IF PARTICIPANT DID RECEIVE THE SALIVA SAMPLE COLLECTION KIT:
Ask if the participant is interested in providing a saliva specimen
Ask if participant has any questions or need any assistance
Ask participant when they plan to mail the kit back
IN CLOSING:
Ask if participant has any questions
Remind participant of your name
Thank them and end call
B-1i. – Email/letter thank you for participating in the biorepository
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent following the completion of the biospecimen collection to thank the participant for participation in the biorepository.
[Date]
Dear [Participant Name]:
Thank you for taking part in the National ALS Biorepository. This Biorepository is being done to increase the number of specimens available for ALS research. A summary of the results of this specimen collection will be posted on the National ALS Registry website. Participants and other interested persons will be able to view the report. We will post a list of studies that used specimens from the Biorepository.
Collecting and storing biological specimens can lead to research. This research can improve clinical care for ALS patients. Many studies into the causes, progression, and prevention of ALS need specimens. Your specimens will be available to ALS Researchers. Data from the National ALS Registry risk factor surveys can help approved researchers using your specimens. If you have not already done so, please consider logging into your Registry account and taking the surveys. Your generous donation will help ALS research in the future.
If you have any questions, please contact Laurie Wagner, the Biorepository Coordinator, by phone at 1-855-874-6912. You can reach her by email at lwagner@secure.mcking.com. Once again, we thank you for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Reading level 11.2 (changing biorepository to study 7
B-1j. – Phone script to provide additional blood (and other biospecimen) collection options
This is a phone script for making calls to individuals who received a blood information packet. The purpose of this call is to determine if the potential participant would like to participate in the Biorepository and provide them with additional blood collection options. If the potential participant agrees to one of the blood collection options consent will be obtained through a thorough review of the consent form (see script B1b). Once the potential participant agrees and decides on the collection preference, appropriate arrangements will be made to collect biospecimens.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF potential PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
If person asks on phone for your contact information leave your name and phone number only
Thank them and end call
IF potential PARTICIPANT IS AVAILABLE:
Introduce yourself and let the potential participant know that you are calling on behalf of the National ALS Biorepository
Ask if he/she received the information packet
Inform potential participant that you are calling to determine whether he/she is interested in participating in the biorepository and to discuss additional blood collection options.
Ask potential participant if they have had a chance to review the information packet and have a copy available at this time
a) If potential participant does not have a copy of the information packet available and is interested in the biorepository:
Provide a brief explanation of additional blood collection options
Inform the potential participant that because of the pandemic, we have other blood collection options in addition to the blood collection at the potential participant’s home by a phlebotomist.
Inform the potential participant that the blood sample can be collected during a regularly scheduled doctor’s office visit OR by an existing home healthcare worker that the potential participant has currently employed.
Offer to send or email another copy and reschedule a time to call back (depending on their mode of contact.
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
Verify mailing and email address.
Remind potential participant to review consent form and to have a copy available for the next call
Thank them and end call
If potential participant does have a copy of the information packet but has not reviewed it:
Allow potential participant time to review information while you wait on the phone
Provide a brief description of the additional blood collection options
Inform the potential participant that because of the pandemic, in addition to the sending a phlebotomist to your home to collect your blood, we have added two alternative blood collection options.
Inform the potential participant that the blood sample can be collected during a regularly scheduled doctor’s office visit OR by an existing healthcare worker that is currently employed by the potential participant.
Ask if potential participant wants to continue to participate in the biorepository
If potential participant does have a copy available and has reviewed information packet:
Provide a brief explanation of alternative blood collection options
Inform the potential participant that because of the pandemic, in addition to the sending a phlebotomist to your home to collect your blood, we have added two alternative blood collection options
Inform the potential participant that the blood sample can be collected during a regularly scheduled doctor’s office visit OR by an existing healthcare worker that is currently employed by the potential participant.
Ask if potential participant wants to continue to participate in the biorepository
IF potential PARTICIPANT DOES NOT WANT TO CONTINUE TO PARTICIPATE IN THE BIOREPOSITORY:
Remind potential participant of your name
Thank them and end call
IF POTENTIAL PARTICIPANT DOES WANT TO CONTINUE TO PARTICIPATE IN THE BIOREPOSITORY:
Ask which blood collection option the potential participant prefers. Update the tracking spreadsheet.
If potential participant has previous information packet/consent form
Inform the potential participant that a new information packet/consent will be mailed/emailed to their address.
Inform the potential participant that you will contact them within one week to complete next steps.
Refer to B-1b script to consent participant and schedule the appointment.
Schedule appointment based on collection option selected by participant.
If potential participant has a current information packet/consent form
Refer to B-1b script to consent participant and schedule the appointment.
Schedule appointment based on collection method selected by participant.
B-1k. – Phone script used when a participant who has provided a saliva specimen is contacted again to collect blood (and other specimens)
This is a phone script for making calls to participants who received a saliva collection information packet. The purpose of this call is to determine if the participant would like to provide blood (and other specimens) to the Biorepository and they have already provided a saliva specimen to the Biorepository. If the participant would like to donate blood at this time, a blood information packet would be sent to the participant to review. A follow up call will be made in one week and script B1b will be used.
INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with participant
IF
PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD
TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
If person asks on phone for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository.
Tell participant that you are calling because they have provided a saliva sample to the Biorepository and want to ask if they would be interested in providing a blood sample as well.
If participant says No, thank them and end the call.
If participant says Yes:
Verify mailing address.
Tell participant you will mail a blood (and other biospecimen) information packet and follow up with them in one week to continue the consent process.
Refer to script B-1b to continue consent process.
B-1l. – Phone script for when a potential participant has agreed to a blood (and other specimens) collection is contacted again and no collection took place
This is a phone script for making calls to individuals who have received a blood collection information packet and have agreed to give a blood specimen. The purpose of this call is to determine if the potential participant would like to donate blood (and other specimens) to the Biorepository. If the potential participant is still interested, then refer to script B1b for the consent process.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant.
IF
POTENTIAL PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD
TIME FOR NEXT CONTACT:
Day/Date:
____________________________
Time:
________________________________
If person asks on phone for your contact information leave your name and phone number only
Thank them and end call
IF POTENTIAL PARTICIPANT IS AVAILABLE:
Introduce yourself and let the potential participant know that you are calling on behalf of the National ALS Biorepository.
Tell potential participant that you are calling because they have previously expressed interest in taking part in the biorepository and you are calling to check if they are still interested in providing a blood sample.
If potential participant says No, thank them and end the call.
If potential participant says Yes:
If potential participant has a previous consent form or has sent in a consent form that has not been signed in the current calendar year, verify current mailing address, mail potential participant a new information packet and follow up with potential participant in one week. Refer to script B-1b to continue consent process at that time.
If potential participant has current consent form, continue to script B-1b to continue consent process.
Appendix B-2. Other participant materials
B-2a.—Fact Sheets
B-2a(1) – Fact Sheet Full collection
National
Amyotrophic Lateral Sclerosis (ALS) Registry Biorepository Fact
Sheet: All Biospecimens
What is the National ALS Biorepository about? |
The National ALS Biorepository (Biorepository) was created by the Agency for Toxic Substances and Disease Registry (ATSDR) to collect, store, and share samples from participants in the National ALS Registry. |
|
What is a Biorepository? |
A biorepository is a facility that collects and stores samples of biological material. This could include blood, urine, tissue, cells, DNA, and proteins. Some medical information may also be stored along with a written consent form. These samples will be used for future research.
|
|
Who is managing the Biorepository? |
This Biorepository is funded by ATSDR. ATSDR is a federal public health agency located in Atlanta, Georgia. McKing Consulting Corporation (McKing) has been awarded a contract to collect and distribute specimens for the Biorepository. |
|
Why is this Biorepository important? |
The specimens in this biorepository will complement the Registry’s epidemiologic data. It will also add to the total numbers of biological specimens available for research on ALS. The National ALS Biorepository will differ from others already in existence because it will include ALS patients from the entire country. In addition, it will not be limited to those with specific exposures or clinical findings. |
|
Who can take part in the Biorepository? |
Persons with ALS enrolled in the National ALS Registry. |
|
What information about me will be collected? |
You will be asked to sign a consent form and answer a few brief questions. Then the phlebotomist will draw blood, clip hair and nail specimens, and you will provide a urine specimen. The samples will be stored for future research on ALS. |
|
Where will the specimens be taken? |
We will contact you to set up a time for a trained professional to come to your home and draw your blood and collect the other specimens. |
|
Is there an alternative collection process? |
Yes. If you have a scheduled doctor’s visit or a home health care worker already coming to your home, we can coordinate with them to collect your specimens. |
|
Is there any risk to me? |
Very little. We will try to make you as comfortable as possible but taking your blood may hurt a little. You will feel a slight “pinch “when the needle is put in. You may feel some discomfort or see a small bruise where the blood was drawn. There is no risk for the other specimens being collected. |
|
Is there any benefit to me? |
There is no direct benefit to you. The Biorepository will collect, store and process biospecimen samples. However, with further research, your samples may help us to better understand ALS in the future. |
|
Will the information I tell you be kept private? |
Yes. Just like when you talk to your doctor, everything you tell us will be kept private to the extent allowed by law. Any information with your name on it will be kept in a locked area. Only authorized employees will be able to look at this information. Results will be posted on the National ALS Registry website. Individuals will not be identified. |
|
What will happen after I provide specimens? |
Results of the Biorepository will be summarized and posted on the National ALS Registry website for all participants and other interested persons to view. Individuals will not be identified. ATSDR will keep your specimens for future ALS research. |
|
Is there anything I need to do to prepare for the day I have the specimens taken? |
Yes.
|
|
Is there anything I need to do on the day I have the specimens taken? |
Yes.
|
|
How long will it take? |
Each specimen collection home visit should take about 30 minutes. |
|
Is there any cost to me for the specimen collection? |
No. There is no charge for the specimens being collected. |
|
Do I have to participate in the Biorepository? |
No. Taking part in the Biorepository is completely voluntary. You can refuse to answer any question for any reason. You can also refuse to provide a blood sample. You may choose to leave the Biorepository at any time even after signing the consent form. There is no penalty for leaving the Biorepository. Your decision will have no impact on your medical care, other services provided by your neurologist, or your participation in the National ALS Registry. |
|
For more information about the Biorepository, contact Laurie Wagner, MPH, Biorepository Coordinator Toll-free: 1-855-874-6912 Email: lwagner@secure.mcking.com Website: wwwn.cdc.gov/als/alsBioRegistry.aspx
|
|
|
Reading level 8.1
B-2a(2).—Fact Sheet without Hair and Nails
This will serve as a FAQ document. It will be included in the packet of information mailed to selected registry enrollees that have indicated interest in participating in the biorepository. It will also be emailed or mailed to any enrollees that ask for more information about the biorepository.
National
Amyotrophic Lateral Sclerosis (ALS) Registry Biorepository Fact
Sheet: Biospecimens
What is the National ALS Biorepository about? |
The National ALS Biorepository (Biorepository) was created by the Agency for Toxic Substances and Disease Registry (ATSDR) to collect, store, and share samples from participants in the National ALS Registry. |
|
What is a Biorepository? |
A biorepository is a facility that collects and stores samples of biological material. This could include blood, urine, tissue, cells, DNA, and proteins. Some medical information may also be stored along with a written consent form. These samples will be used for future research.
|
|
Who is managing the Biorepository? |
This Biorepository is funded by ATSDR. ATSDR is a federal public health agency located in Atlanta, Georgia. McKing Consulting Corporation (McKing) has been awarded a contract to collect and distribute specimens for the Biorepository. |
|
Why is this Biorepository important? |
The specimens in this biorepository will complement the Registry’s epidemiologic data. It will also add to the total numbers of biological specimens available for research on ALS. The National ALS Biorepository will differ from others already in existence because it will include ALS patients from the entire country. In addition, it will not be limited to those with specific exposures or clinical findings. |
|
Who can take part in the Biorepository? |
Persons with ALS enrolled in the National ALS Registry. |
|
What information about me will be collected? |
You will be asked to sign a consent form and answer a few brief questions. Then the phlebotomist will draw blood and you will provide a urine specimen. The samples will be stored for future research on ALS. |
|
Where will the specimens be taken? |
We will contact you to set up a time for a trained professional to come to your home and draw your blood and collect the other specimens. |
|
Is there an alternative collection process? |
Yes. If you have a scheduled doctor’s visit or a home health care worker already coming to your home, we can coordinate with them to collect your specimens. |
|
Is there any risk to me? |
Very little. We will try to make you as comfortable as possible but taking your blood may hurt a little. You will feel a slight “pinch “when the needle is put in. You may feel some discomfort or see a small bruise where the blood was drawn. There is no risk for the other specimens being collected. |
|
Is there any benefit to me? |
There is no direct benefit to you. The Biorepository will collect, store and process biospecimen samples. However, with further research, your samples may help us to better understand ALS in the future. |
|
Will the information I tell you be kept private? |
Yes. Just like when you talk to your doctor, everything you tell us will be kept private to the extent allowed by law. Any information with your name on it will be kept in a locked area. Only authorized employees will be able to look at this information. Results will be posted on the National ALS Registry website. Individuals will not be identified. |
|
What will happen after I provide specimens? |
Results of the Biorepository will be summarized and posted on the National ALS Registry website for all participants and other interested persons to view. Individuals will not be identified. ATSDR will keep your specimens for future ALS research. |
|
Is there anything I need to do on the day I have the specimens taken? |
Yes.
|
|
How long will it take? |
Each specimen collection home visit should take about 30 minutes.
|
|
Is there any cost to me for the specimen collection? |
No. There is no charge for the specimens being collected. |
|
Do I have to participate in the Biorepository? |
No. Taking part in the Biorepository is completely voluntary. You can refuse to answer any question for any reason. You can also refuse to provide a blood sample. You may choose to leave the Biorepository at any time even after signing the consent form. There is no penalty for leaving the Biorepository. Your decision will have no impact on your medical care, other services provided by your neurologist, or your participation in the National ALS Registry. |
|
For more information about the Biorepository, contact Laurie Wagner, MPH, Biorepository Coordinator Toll-free: 1-855-874-6912 Email: lwagner@secure.mcking.com Website: wwwn.cdc.gov/als/alsBioRegistry.aspx
|
Reading level 8.6 |
|
B-2a(3).—Fact Sheet Saliva only
This will serve as a FAQ document. It will be included in the packet of information mailed to selected registry enrollees that have indicated interest in participating in the biorepository. It will also be emailed or mailed to any enrollees that ask for more information about the biorepository.
National
Amyotrophic Lateral Sclerosis (ALS) Registry Biorepository Fact
Sheet: Saliva What is the National ALS Biorepository about? |
The National ALS Biorepository (Biorepository) was created by the Agency for Toxic Substances and Disease Registry (ATSDR) to collect, store, and share samples from participants in the National ALS Registry. |
|
What is a Biorepository? |
A biorepository is a facility that collects and stores samples of biological material. This could include blood, urine, tissue, cells, DNA, and proteins. Some medical information may also be stored along with a written consent form. These samples will be used for future research.
|
|
Who is managing the Biorepository? |
This Biorepository is funded by ATSDR. ATSDR is a federal public health agency located in Atlanta, Georgia. McKing Consulting Corporation (McKing) has been awarded a contract to collect and distribute specimens for the Biorepository. |
|
Why is this Biorepository important? |
The specimens in this biorepository will complement the Registry’s epidemiologic data. It will also add to the total numbers of biological specimens available for research on ALS. The National ALS Biorepository will differ from others already in existence because it will include ALS patients from the entire country. In addition, it will not be limited to those with specific exposures or clinical findings. |
|
Who can take part in the Biorepository? |
Persons with ALS enrolled in the National ALS Registry. |
|
What information about me will be collected? |
You will be asked to sign a consent form. Your sample will be stored for future research on ALS. |
|
Where will the specimens be taken? |
You will provide a saliva specimen in your home at your convenience. |
|
Is there any risk to me? |
There is no risk when providing a saliva specimen. |
|
Is there any benefit to me? |
There is no direct benefit to you. The Biorepository will collect, store and process biospecimen samples. However, with further research, your samples may help us to better understand ALS in the future. |
|
Will the information I tell you be kept private? |
Yes. Just like when you talk to your doctor, everything you tell us will be kept private to the extent allowed by law. Any information with your name on it will be kept in a locked area. Only authorized employees will be able to look at this information. Results will be posted on the National ALS Registry website. Individuals will not be identified. |
|
What will happen after I provide specimens? |
Results of the Biorepository will be summarized and posted on the National ALS Registry website for all participants and other interested persons to view. Individuals will not be identified. ATSDR will keep your specimens for future ALS research. |
|
Is there anything I need to do before donating specimens? |
Do not eat, drink, smoke or chew gum for 30 minutes before giving your saliva sample. |
|
How will I provide my specimen? |
You will be asked to spit into a small funnel that will be mailed to your home. Instructions for providing the saliva specimen will be mailed with the kit. |
|
How long will it take? |
Giving a saliva specimen should take about 10 minutes. |
|
Is there any cost to me for the specimen collection? |
No. There is no charge for the specimens being collected. |
|
Do I have to participate in the Biorepository? |
No. Taking part in the Biorepository is completely voluntary. You can refuse to answer any question for any reason. You can also refuse to provide a saliva specimen. You may choose to leave the Biorepository at any time even after signing the consent form. There is no penalty for leaving the Biorepository. Your decision will have no impact on your medical care, other services provided by your neurologist, or your participation in the National ALS Registry. |
|
For more information about the Biorepository, contact Laurie Wagner, MPH, Biorepository Coordinator Toll-free: 1-855-874-6912 Email: lwagner@secure.mcking.com Website: wwwn.cdc.gov/als/alsBioRegistry.aspx
|
|
|
Reading level 9.1
B-2b. – Instructions for opening the specimen kit
These guidelines will provide instructions to participants receiving and opening the specimen collection kit from the laboratory. This will be sent with Email/Letter to interested PALS (B-1c.).
National ALS Biorepository
Guidelines for opening Collection Kit
Step 1: Open the outer shipping box.
Step 2: Take the lid off of the Styrofoam container.
Step 3: Remove the yellow bag that contains the Urine Collection Kit.
Step 4: Remove the two cooling gel packs.
Step 5: Once the Urine Collection Kit and cooling gel packs are removed, replace the Styrofoam lid and set aside the Collection Kit and outer shipping box. The Collection Kit will not be used again until the phlebotomist arrives, the day of your specimen collection appointment.
Step 6: Open the yellow bag when you are ready to provide your urine sample. It is best to get the sample when you first get up in the morning on the day of your appointment.
The Urine Collection Kit should contain the following:
Plastic collection container
Disposable gloves
Towelette
Step 7: Follow the directions included with the urine collection kit.
Step 8: Place the urine container back in the resealable yellow bag and place the bag and cooling gel packs in the refrigerator.
Step 9: Call a member of the National ALS Biorepository team at 1-855-874-6912 if you have questions.
Reading Level 8.7
B-2b.1 – Instructions for opening the specimen kit when not collecting urine
These guidelines will provide instructions to participants receiving and opening the specimen collection kit from the laboratory when urine is not being collected. This will be sent with Email/Letter to interested PALS (B-1c.).
National ALS Biorepository
Guidelines for opening Collection Kit
Step 1: Open the outer shipping box.
Step 2: Take the lid off of the Styrofoam container.
Step 3: Remove the two cooling gel packs.
Step 4: Once the cooling gel packs are removed, replace the Styrofoam lid and set aside the Collection Kit and outer shipping box (Do not put box in refrigerator). The Collection Kit and outer shipping box will not be used again until the phlebotomist arrives, the day of your specimen collection.
Step 5: Place cooling gel packs in the refrigerator (Do not put in freezer).
Step 6: Call a member of the National ALS Biorepository team at 1-855-874-6912 if you have questions.
Reading level 8.9
B-2c. – Instructions for participant urine collection
These guidelines provide instructions to the participant on completing the urine collection prior to the arrival of the phlebotomist. This document will be included in participant information packet as well as including in the urine collection package that will arrive with the collection kit from the laboratory.
OPEN THE URINE COLLECTION KIT:
Open the urine collection cup package. DO NOT REMOVE the label from the top of the Urine Collection Cup. There is a needle under the label.
Put the yellow-top tubes to the side for the phlebotomist to use
Remove the sterile wipe
PREPARE FOR URINE COLLECTION:
Wash hands thoroughly with soap and water
Unscrew the blue cap
Place the blue cap on the counter with “straw” facing upwards. Do not touch the inside of cap or the straw.
FEMALE COLLECTION INSTRUCTIONS:
Separate the folds of skin around the urinary opening.
Using the sterile wipe, clean the area using front-to-back strokes
Discard the wipe
Void a small amount of urine into the toilet
As you continue to urinate, bring the collection cup into the midstream to collect the urine sample
Do not touch the inside or lip of the cup
Replace the blue cap onto the collection cup
Place cup inside bag and place in the refrigerator
Give to the phlebotomist when she arrives
MALE COLLECTION INSTRUCTIONS:
Pull foreskin back (if necessary)
Using the sterile wipe, clean the end of the penis by rubbing in a circular motion starting at the urethra opening and moving outwards
Discard the wipe
Void a small amount of urine into the toilet
As you continue to urinate, bring the collection cup into the midstream to collect the urine sample
Do not touch the inside or lip of the cup
Replace the blue cap onto the collection cup
Place cup inside bag and place in the refrigerator
Give to the phlebotomist when she arrives
Reading Level 5.1
B-2d. – Instructions for saliva collection
These guidelines will provide instructions to participants completing the self-collected saliva collection. This will be included in the saliva collection kit package that is shipped to their home.
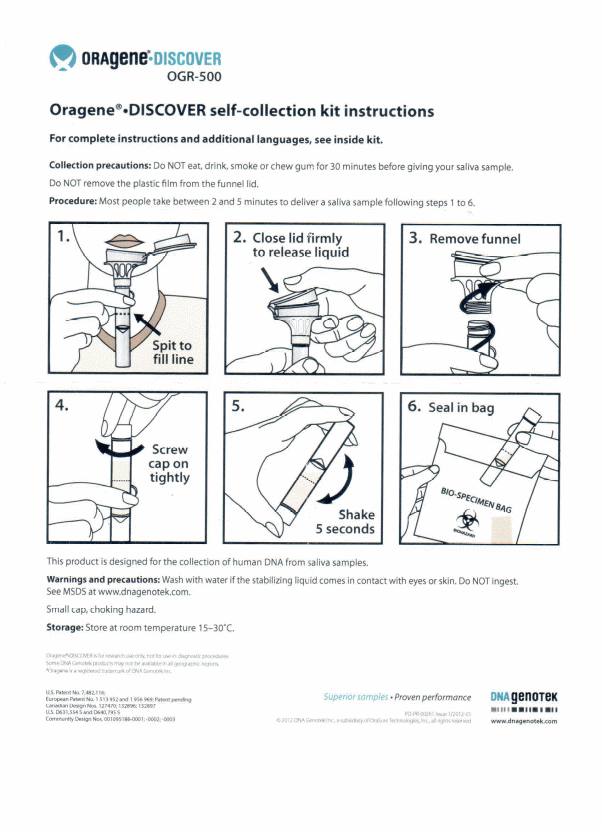
National ALS Biorepository Consent Form (Biospecimens)
Why am I being asked?
You are being asked to take part in the biorepository because you are enrolled in the National ALS Registry and have told us you are interested in learning more about the biorepository. The biorepository is a new part of the National ALS Registry maintained by the Agency for Toxic Substances and Disease Registry (ATSDR). We ask that you read this form and ask any questions you may have before agreeing to be in the biorepository. McKing is the company that was awarded a contract to manage the biorepository. The biorepository Senior Scientist is Dr. Wendy E. Kaye.
Taking part in this biorepository is voluntary. You should feel free to ask the researchers any questions you may have. Your decision whether or not to take part will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
What is the purpose of this biorepository?
Page
1 of 4
Approximately 675 people will be asked to contribute specimens to the biorepository each year.
What are the procedures involved?
You will be asked to have specimens collected from you at your home. This should take about 30 minutes.
You are being asked to donate specimens. We will make an appointment for a phlebotomist to come to your home to collect specimens at a time convenient for you or you can ask a qualified home health worker already coming to your home to collect the specimens, or you can take your kit to an already scheduled doctor’s appointment and have their staff do the collection. We will ask you to donate the specimen types checked below.
A urine specimen collection kit with directions will be sent to your home before your appointment. You will be asked to provide a urine specimen (9 ml) before the end of your appointment. The best time to give a urine specimen is when you first get up in the morning on the day of your appointment.
A blood sample will be drawn from your arm with a needle by a trained phlebotomist. There will be 5 tubes of blood taken (about 40 ml or 2.5 tablespoons).
Fingernail clippings will be collected by the phlebotomist from all ten (10) fingers using a stainless steel nail clipper.
Hair clippings will be collected by the phlebotomist on the day of your appointment. The hair specimen collection will be about 120 strands. These will be clipped from the back of your head, near your neck, and under your other hair.
Saliva samples (2 ml) will be taken only if you are unable to give blood or if there is a problem with the blood. A saliva sample kit with directions will be mailed to your home and you will be asked to collect the saliva sample yourself.
Additional Instructions:
Drink plenty of water on the day of your blood draw.
You will be sent a cup to collect a urine specimen. We ask that you collect this the morning before your appointment.
Do not cut your hair for at least 1 week before your appointment, if we are collecting hair.
Hair should be free of all gels, oils and hair creams or sprays prior to sample collection. The hair to be collected should be untreated (not permed, dyed or bleached).
Do not cut your fingernails for at least 1 week before your appointment. Please remove nail polish for the day of your appointment, if we are collecting fingernails.
Tissue Banking
Page
2 of 4
Researchers can ask to use specimens from the biorepository for research related to ALS. We do not know what types of research will be done. These studies will likely examine the DNA and RNA (genetic material) and other material from cells in the tissues. Researchers must provide a detailed plan of the study. This plan must be approved by ATSDR and the ethics committees at the researchers’ institutions. Your specimens along with others will be provided to approved researchers. These specimens will not have your name on them. The specimens will include information about you such as your age or city where you lived. They may also request other data you gave the National ALS Registry. We will limit the amount of information about you to reduce the chances that you will be identified.
Results of research using these specimens will be available on the National ALS Registry website.
What are some of the risks and discomforts that may happen to people who are in this biorepository?
You will feel a slight “pinch” when the needle is put in to draw your blood. You may feel some pain or see a small bruise where the blood was drawn. There is little risk for the other specimens being collected.
There is a slight risk that someone could get access to the data we have stored about you. If information about you does leak out, ATSDR will not be able to guarantee that it will be protected.
There is a very small chance that genetic research in the future could give genetic information that could be used to identify you.
Are there benefits to taking part in this biorepository?
You will not directly benefit from taking part. It is hoped that knowledge gained from this research may benefit others with ALS in the future.
What other options are there?
You have the option to not to take part in this biorepository.
What about privacy and confidentiality?
If you take part in the biorepository, we will not have access to your private medical records.
The people who will know that you take part in this biorepository are members of the biorepository team, individuals who may have access to your informed consent document due to their job function with the National ALS Biorepository, and people processing your specimens.
Information that identifies you and the consent form signed by you will be looked at by the ATSDR.
A possible risk of the biorepository is that people outside of the biorepository find out you are taking part in the biorepository or learn information about you and your health. Your specimens will be stored with a code. Your name will not be on the container.
Page
3 of 4
When the results of the biorepository are published or discussed at meetings, no information will be included that would reveal your identity
Will my family be told biorepository results?
Your family will not be told of any new findings that come to light during the course of using your specimens. We will not tell you or your family any genetic testing results from research studies. ATSDR will post study results on the National ALS Registry website.
What are the costs for participating in this biorepository?
There is no cost for participating in this biorepository. You will not be charged for any lab tests.
Will I be reimbursed for any of my expenses or paid for my participation in this biorepository?
You will not be paid to take part in this biorepository.
Will my cells, tissues, blood, or other biological materials be used to develop commercial products?
It is possible that a commercial product may be developed from the tissue or blood samples collected as part of this biorepository. The nature of the research means that your sample is only one of many that will lead to this product and your sample will not have your name on it. You and your family will not profit financially from such a product. You will have no legal rights to any discovery or invention that either directly or indirectly results from the use of your specimens, individual information, or information from your samples.
Cells obtained from your body may be used to establish a cell line which may be shared in the future with other researchers and which may be of commercial value. A cell line is one which will grow indefinitely in the laboratory. Cell lines may be useful because of the characteristics of the cells and/or the products they may produce.
Can I withdraw or be removed from the biorepository?
Taking part in this biorepository is voluntary. If you choose not to take part or decide to withdraw your consent and stop taking part, this will not affect your relationship with National ALS Registry, or other benefits to which you are otherwise entitled.
You have the right to leave the biorepository at any time without penalty. If you withdraw from the biorepository, we will destroy any of your remaining samples. However, we will not be able to remove your samples that have already been used or shared with researchers.
Who should I contact if I have questions regarding the biorepository? Who should I contact if I wish to voice concerns or complaints? Who can I talk to about my rights or want to withdraw my samples?
If you have questions, concerns, or complaints, think the biorepository has hurt you, or if you want to withdraw your samples, you can talk to Wendy E. Kaye, Ph.D., Senior Scientist or Laurie Wagner, MPH, Biorepository Coordinator at 1-855-874-6912.
If you want to speak with someone who is not directly involved in this biorepository, or have questions about your rights, or wish to voice questions, concerns or complaints, you may contact the CDC Human Research Protection Office at 1-800-584-8814.
Remember
Taking part in this biorepository is voluntary. Your decision whether or not to take part will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
Subjects’ Rights:
I have read the above information. I have discussed this biorepository with the person obtaining consent, been given an opportunity to ask questions and my questions have been answered to my satisfaction. I agree to participate in this biorepository. I will be given a copy of this signed and dated consent form.
__________________________________________
Print Subject name
Signature of Subject Date

If participant is physically unable to sign the consent form, please complete the following
Witness Signature Date
I, _____________________, witnessed that _________________ was explained the consent form and has agreed to take part in this biorepository. Due to the progression of the disease, the participant is physically unable to sign the consent form.
Page
4 of 4
National ALS Biorepository Consent Form (Saliva)
Why am I being asked?
You are being asked to take part in the biorepository because you are enrolled in the National ALS Registry and have told us you are interested in learning more about the biorepository. The biorepository is a new part of the National ALS Registry maintained by the Agency for Toxic Substances and Disease Registry (ATSDR). We ask that you read this form and ask any questions you may have before agreeing to be in the biorepository. McKing is the company that was awarded a contract to manage the biorepository. The biorepository Senior Scientist is Dr. Wendy E. Kaye.
Taking part in this biorepository is voluntary. You should feel free to ask the researchers any questions you may have. Your decision whether or not to take part will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
What is the purpose of this biorepository?
Page
1 of 4
Approximately 675 people will be asked to contribute specimens to the biorepository each year.
What are the procedures involved?
You will be asked to collect saliva from which DNA can be extracted. We will send a special kit to your home. This should take about 10 minutes.
Tissue Banking
Page
2 of 4
Researchers can ask to use specimens from the biorepository for research related to ALS. We do not know what types of research will be done. These studies will likely examine the DNA and RNA (genetic material) and other material from cells in the tissues. Researchers must provide a detailed plan of the study. This plan must be approved by ATSDR and the ethics committees at the researchers’ institutions. Your specimens along with others will be provided to approved researchers. These specimens will not have your name on them. The specimens will include information about you such as your age or city where you lived. They may also request other data you gave the National ALS Registry. We will limit the amount of information about you to reduce the chances that you will be identified.
Results of research using these specimens will be available on the National ALS Registry website.
What are some of the risks and discomforts that may happen to people who are in this biorepository?
There is little risk for collecting saliva.
There is a slight risk that someone could get access to the data we have stored about you. If information about you does leak out, ATSDR will not be able to guarantee that it will be protected.
There is a very small chance that genetic research in the future could give genetic information that could be used to identify you.
Are there benefits to taking part in this biorepository?
You will not directly benefit from taking part. It is hoped that knowledge gained from this research may benefit others with ALS in the future.
What other options are there?
You have the option to not to take part in this biorepository.
What about privacy and confidentiality?
If you take part in the biorepository, we will not have access to your private medical records.
The people who will know that you take part in this biorepository are members of the biorepository team, individuals who may have access to your informed consent document due to their job function with the National ALS Biorepository, and people processing your specimens.
Information that identifies you and the consent form signed by you will be looked at by the ATSDR.
A possible risk of the biorepository is that people outside of the biorepository find out you are taking part in the biorepository or learn information about you and your health. Your specimens will be stored with a code. Your name will not be on the container.
Page
3 of 4
When the results of the biorepository are published or discussed at meetings, no information will be included that would reveal your identity
Will my family be told biorepository results?
Your family will not be told of any new findings that come to light during the course of using your specimens. We will not tell you or your family any genetic testing results from research studies. ATSDR will post study results on the National ALS Registry website.
What are the costs for participating in this biorepository?
There is no cost for participating in this biorepository. You will not be charged for any lab tests.
Will I be reimbursed for any of my expenses or paid for my participation in this biorepository?
You will not be paid to take part in this biorepository.
Will my cells, tissues, blood, or other biological materials be used to develop commercial products?
It is possible that a commercial product may be developed from your saliva sample collected as part of this biorepository. The nature of the research means that your sample is only one of many that will lead to this product and your sample will not have your name on it. You and your family will not profit financially from such a product. You will have no legal rights to any discovery or invention that either directly or indirectly results from the use of your specimens, individual information, or information from your samples.
Can I withdraw or be removed from the biorepository?
Taking part in this biorepository is voluntary. If you choose not to take part or decide to withdraw your consent and stop taking part, this will not affect your relationship with National ALS Registry, or other benefits to which you are otherwise entitled.
You have the right to leave the biorepository at any time without penalty. If you withdraw from the biorepository, we will destroy any of your remaining samples. However, we will not be able to remove your samples that have already been used or shared with researchers.
Who should I contact if I have questions regarding the biorepository? Who should I contact if I wish to voice concerns or complaints? Who can I talk to about my rights or want to withdraw my samples?
If you have questions, concerns, or complaints, think the biorepository has hurt you, or if you want to withdraw your samples, you can talk to Wendy E. Kaye, Ph.D., Senior Scientist or Laurie Wagner, MPH, Biorepository Coordinator at 1-855-874-6912.
If you want to speak with someone who is not directly involved in this biorepository, or have questions about your rights, or wish to voice questions, concerns or complaints, you may contact the CDC Human Research Protection Office at 1-800-584-8814.
Remember
Taking part in this biorepository is voluntary. Your decision whether or not to take part will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
Subjects’ Rights:
I have read the above information. I have discussed this biorepository with the person obtaining consent, been given an opportunity to ask questions and my questions have been answered to my satisfaction. I agree to participate in this biorepository. I will be given a copy of this signed and dated consent form.
___________________________________
Print Subject name
Signature of Subject Date

If participant is physically unable to sign the consent form, please complete the following
Witness Signature Date
I, _____________________, witnessed that _________________ was explained the consent form and has agreed to take part in this biorepository. Due to the progression of the disease, the participant is physically unable to sign the consent form.
Page
4 of 4
B-4a. Urine
Urine collection will be a spot random collection on the day of the appointment for the blood draw. The urine collection kit will be mailed to the participant prior to the visit with instructions on how to collect the specimen. Participants will be encouraged to collect the specimen in the morning and provide it to the phlebotomist when he/she comes for the home visit or it may be collected at the time of the visit.
Upon arrival, the phlebotomist will ask the participant if he/she has already obtained the urine specimen. If not, the specimen will be collected at the end of the visit.
Participants will be provided a prescreened (for heavy metals and phthalates) high-density polypropylene 150-mL urine collection cup with screw cap. The prescreening will be lot tested for heavy metals and phthalates. Only lots that test negative will be used. The urine specimen is collected directly into the container, which is then securely sealed and placed into a resealable plastic bag provided with the kit to provide secondary containment. If the participant collects the specimen before the phlebotomy appointment, it should be placed into the home refrigerator until the time of the visit, to minimize degradation.
B-4b. Blood
Blood will be collected using a 21-gauge x ¾” or 23-gauge x ¾” butterfly needle blood collection set, with 12” tubing and safety-lock for the needle. (*Saliva samples will not be collected by phlebotomists; self-collection of saliva will be used only when blood collection fails.)
All lots of 6-mL K2EDTA Vacutainer should be pre-screened before use in this biorepository by lot-testing for background heavy metals contamination. This screening includes Pb, Cd, Hg, Se, and Mn.
ORDER OF DRAW OF BLOOD COLLECTION TUBES:
10-mL K2 EDTA (B-D 366643 Vacutainer™, lavender Hemogard™ closure, plastic) for DNA, proteins, red cell lipids
6.0-mL K2 EDTA (B-D 367856 Vacutainer™, lavender Hemogard™ closure, plastic) for heavy metals (Pb, Cd, Hg)
10-mL plain (B-D 366430 Vacutainer™, no anticoagulant, silicone-coated, red conventional closure, glass)
1 X 8-mL CPT blood Tube for cell line creation
1 or 2 X 2.5-mL PAXgene™ blood RNA Tube (B-D 762165, plastic, Hemogard™ closure, 6.9 mL additive stabilizer)
Collecting the 6.0-mL EDTA in second order allows the butterfly needle line to be flushed of potential heavy metal contamination beforehand. The PAXgene tubes are always collected last because they contain 6.9 mL of stabilizer.
When blood is collected from a participant, the Vacutainers™ are inverted 10 times to mix the anti-coagulant/preservative with the whole blood (or to activate clotting in the red-top tubes with no anticoagulant). Using the butterfly collection device allows the phlebotomist to perform critical thorough mixing of the previous tube while the next tube is being collected, and it minimizes any discomfort of changing tubes while the needle is still in the subject’s vein.
After collection from each participant, these specimens will be carefully packed in Styrofoam-insulated shippers with properly conditioned reusable cold-packs to maintain a temperature between 4-8 °C for overnight shipping by express courier to Fisher BioServices. Dry ice cannot be used because exposure to freezing temperatures could hemolyze red blood cells and have an adverse effect on plasma or serum –based analyses performed in the future. The specimen will be packaged according to current IATA regulations for clinical samples as “exempt human specimens”, packed in leak-proof primary and secondary packaging with enough absorbent material to soak up entire contents of the bag. It must include an itemized list of contents between the secondary packaging and outer packaging. Each shipper should also contain a reusable “data logger” to monitor temperatures during shipment.
B-4c. Hair
Use the specially cleaned surgical scissors, plastic comb, and aluminum hair clips provided in the individual specimen collection kit. Wear disposable nitrile powder-free gloves while handling hair specimens.
Collect the hair samples as follows:

Collect samples from the nape area (diagram A).
Partition the hair between the ears (diagram B).
Fasten the hair above the ears and hold the upper hair out of the way with aluminum clips (diagram B).
From each of 8-10 sites at the nape area (diagram C), gather 15-20 strands of hair. Hold the end of the hair, and cut the hair as close to the scalp as possible.
For each cutting of long hair, cut off the 2 inches of hair that had been closest to the scalp and store this sample in the labeled resealable container provided.
If hair is extremely short, cut as available and note very short hair length in subject record.
Discard the remaining cut hair above the lower 2 inches closest to the scalp.
Be sure to complete the questionnaire of subject use of hair care products (dyes and permanents often contain heavy metals such as mercury, dandruff shampoos can contain selenium and zinc, ethnic products can also contain heavy metals, etc.).
B-4d. Fingernails
The subject will have been notified in a previous mailing that fingernail clippings will be requested for future study. If he/she has forgotten and has recently trimmed his/her nails too short to permit additional clipping, please note this problem in the subject record.
Use the specially cleaned nail clippers provided in the collection kit. Examine nails to make sure they are free of polish;
If fingernails have any nail polish on them (even clear), please ask if it can be removed.
If the participant agrees, use nail polish remover and then soap & water to wash nails prior to clipping. (Document that you had to perform this treatment in your patient record.)
Do not collect nails from any subject unwilling to remove polish.
Please record if they had nail polish present prior to collection.
Collect nail samples from all ten (10) fingers.
Catch all the clippings directly into the prescreened resealable labeled container provided, and then seal the container.
If the subject’s nails are all too short, leave the collection materials and instructions with the participant. Notify McKing that the subject’s nails were too short. McKing will send shipping materials and instructions to the participant.
B-4e. Specimen Processing Form

B-5. Email/letter announcement of group event
Come learn more about the National ALS Registry and the National ALS Biorepository at the _________________ meeting on date at time. We will be meeting at ________________. Representatives from the Registry and Biorepository will be there to answer questions. People enrolled in the Registry can provide a saliva/blood sample (select appropriate sample type) if they are interested.
Please contact ___________ at _________________ if you have questions.
Hope to see you there.
B-6. Biorepository Safety Information
B-6a. Biorepository COVID-19 Safety Procedures and Restart Plan
This document was created to inform the OMB and IRB office of the plan that was created to minimize risk and exposure and safely restart participant sample collections.
Introduction
On March 11, 2020, the Registry paused Biorepository specimen collections to protect the health of the Biorepository participants and members of their household. The Biorepository staff canceled all collection appointments scheduled until further review of the pandemic. Restarting the Biorepository collections will require that precautions be put in place to ensure that staff and participants and their caretakers/household members are protected from COVID-19. This plan is based on interim CDC guidance for non-COVID public health activities that require face-to-face interaction during the COVID-19 pandemic and will be used to outline practices for conducting Biorepository project activities.
Changes in the Biorepository procedures are proposed to reduce or eliminate close contact (within 6 feet) between project staff and participants when possible to prevent the spread of COVID-19 during collection activities. The precautions and personal protective equipment (PPE) recommended in this plan will be reevaluated monthly and more often as needed to ensure they are still in line with CDC COVID-19 guidelines and may be revised to add or remove precautions as needed.
Objectives
Minimize risk of exposure, illness, and spread of disease among staff working with the Biorepository;
Minimize risk of exposure, illness, and spread of disease among Biorepository participants and members of their households; and
Preserve essential functions of the Biorepository.
Schedule Considerations
Scheduling study activities, the Biorepository staff and Phlebotomy companies will consider before appointments are finalized:
The level of local COVID-19 transmission (number of new cases, emergency department visits, and percent positive for testing in each community as available)
Not scheduling appointments in areas with higher-than-average transmission
State and local guidance/mandates
COVID-19 Safety Precautions for the National ALS Biorepository
Staffing: Biorepository staff will oversee the phlebotomists assigned to collect specimens at each location. The Biorepository staff will also oversee collection activities relating to the project operations office, specimen collections, and sample processing. The collections that take place by home healthcare workers and in physician’s offices, the staff with follow the procedures already established by their offices or agencies. The Biorepository staff are not expected to provide in-person support for these activities.
All phlebotomists will be provided clear information about new procedures and PPE (Personal Protective Equipment) requirements by their employer (as described in table below). Phlebotomists will go through collection training and all PPE will be provided to staff prior to initiation of activities. Phlebotomists utilize full PPE, including long sleeves, long pants (or scrubs), closed-toe shoes, and gloves. In addition to the usual PPE used during collections, we are adding extra measures due to COVID-19 which include a cloth face covering or disposable face mask and a face shield that covers eyes, nose, and mouth.
Face coverings are required and meant to protect others in case you are infected. We recommend both the phlebotomists, the participants, and household members present for the specimen collection appointment, wear a cloth face covering or a disposable face mask during the in-home appointment. Cloth face coverings and disposable face masks should not be placed on anyone who has trouble breathing, or is unconscious, incapacitated or otherwise unable to remove the mask without assistance. If participants are unable to wear a cloth face covering or disposable face mask due to medical conditions, the phlebotomist will wear a disposable surgical mask. The Biorepository staff will include at least two disposable face masks in each collection kit that is shipped to the participant for the participant or their family members to wear during appointment.
Screening of Project Phlebotomists, Participants, and Household Members
Phlebotomist screening
Before entering the collection location, phlebotomists will be screened for symptoms associated with COVID-19. Phlebotomist will not be assigned to any collections if they answer yes to having any of the COVID-19 symptoms or traveled in violation of any federal, state, or local guidance or orders, or had close contact with an individual who has any symptoms or travelled in violation in the past 14 days.
Questions on COVID-19 symptoms will include asking about the presence of any of the following in the past 14 days:
Fever or chills
Cough
Shortness of breath or difficulty breathing
Fatigue
Muscle or body aches
Headache
New loss of taste or smell
Sore throat
Congestion or runny nose
Nausea or vomiting
Diarrhea
If any phlebotomist develops any COVID-19 symptoms they will be instructed not to come to work, to inform their supervisor who will notify the Biorepository staff. If a phlebotomist develops symptoms consistent with COVID-19, Biorepository staff will pause that phlebotomists’ collections. The phlebotomist must subsequently test negative for COVID-19, before resuming collection activities. If the phlebotomist tests positive, Biorepository staff will notify any participants who have been in contact with the positive individual.
Participant and Household member screening
The Biorepository staff will ask the participant if in the past 14 days, they have experienced any of the symptoms above or have they had close (within 6 feet) prolonged (10 minutes or more) contact with someone who has had any of the symptoms. In the last 14 days have you traveled in violation of any federal, state, or local guidance or orders, or had close contact with an individual who has. The participants will be asked if they are comfortable scheduling their appointment once the screening questions have been answered. When appointments are scheduled, participants and household members will be informed of screening procedures and be required to wear a cloth face covering or disposable face mask during their appointment. They will also be instructed to notify Biorepository staff to cancel their appointment if they are experiencing COVID-19 symptoms (as defined in the list above). Participants will be asked about symptoms in a reminder call the day before their scheduled appointment.
Additional actions that will be enforced to reduce the potential for COVID-19 transmission include:
Proper hand washing.
Providing disposable face masks for participants who do not have their own face covering upon entry into the home or designated collection location.
Travel Guidelines
Biorepository staff are not normally present during the blood collection and do not interact with the participants during the collection visit. Phlebotomists are the only staff members that meet in person with the participants and other household members.
During travel Biorepository staff will be directed to the considerations to the CDC travel in the US guidelines at https://www.cdc.gov/coronavirus/2019-ncov/travelers/travel-in-the-us.html. Staff will also be provided with the guidance below.
Protect yourself and others during your trip:
Clean your hands often.
Wash your hands often with soap and water for at least 20 seconds especially after you have been in a public place, or after blowing your nose, coughing, or sneezing.
If soap and water are not readily available, use a hand sanitizer that contains at least 60% alcohol. Cover all surfaces of your hands and rub your hands together until they feel dry.
Avoid touching your eyes, nose, and mouth.
Avoid close contact with others.
Keep 6 feet of physical distance from others.
Avoiding close contact is especially important if you are at higher risk of getting very sick from COVID-19.
Wear a cloth face covering or face mask in public.
Cover coughs and sneezes.
Pick up food at drive-throughs, curbside restaurant service, or stores. Do not dine in restaurants if that is prohibited by state or local guidance.
Protective Measures
Specific project activities are shown in the table below with recommended PPE, and additional precautions. All PPE for project phlebotomists will be provided by their organization. See table below.
Activity |
Description |
PPE |
Additional Precautions |
Travel |
Time spent in ride share/public transportation, in airport, on airplane, time spent in public venues while traveling |
Disposable face mask |
|
COVID-19 Screening |
Talk to participants outside of their home or collection location, ask symptom screening questions |
Disposable face mask, disposable gloves |
|
Biological sample collection
|
Collect blood samples |
Phlebotomists: Cloth face covering or face mask, disposable gloves, Phlebotomists will be utilizing full PPE (Personal Protective Equipment), including long sleeves, long pants (or scrubs), closed-toe shoes, gloves and face shields that cover eyes, nose, and mouth. |
|
B-6b. National ALS Biorepository COVID-19 Safety Information Sheet
This document will provide general information to participants that would like to take part in the ALS Biorepository and get samples safely collected during the COVID-19 pandemic. This document will be included in the information packet along with other information that is sent to participants.
Introduction
On March 11, 2020, the Registry paused Biorepository specimen collections to protect the health of those taking part in the Biorepository and members of their households. We are now restarting Biorepository collections and have added precautions to ensure that staff, participants, and their caretakers/household members are protected from COVID-19. This is based on CDC guidance for non-COVID public health activities that require face-to-face interaction during the COVID-19 pandemic.
What you need to know
Changes in the Biorepository procedures have been made to;
Minimize risk of exposure, illness, and spread of disease among staff working with the Biorepository;
Minimize risk of exposure, illness, and spread of disease among those taking part in the Biorepository and members of their households; and
Maintain essential functions of the Biorepository.
COVID-19 Safety
Face coverings are required and meant to protect others in case you are infected. Phlebotomists will wear a disposable surgical mask. The Biorepository staff will include at least two disposable face masks in each collection kit that is shipped to your home. Phlebotomists will go through training and all personal protective equipment will be provided before collections.
COVID-19 Screening
Before each appointment, participants, household members, and phlebotomists will be asked if they have experienced any COVID-19 symptoms in the past 14 days.
When appointments are scheduled, those taking part and household members will be told about the screening procedures. They will also be instructed to notify Biorepository staff to cancel their appointment if they are experiencing COVID-19 symptoms. Participants will be asked again about symptoms in a reminder call the day before their scheduled appointment.
Before entering the home, phlebotomists will be screened for symptoms associated with COVID-19. Phlebotomist will not be assigned to any collections if they answer yes to having any of the COVID-19 symptoms or traveled in violation of any federal, state, or local guidance or orders.
Feel free to contact a member of the National ALS Biorepository by phone at 1-855-874-6912 or by email at alsbiorepository@secure.mcking.com if you have any questions or concerns.
Appendix C: Postmortem Component
Appendix C-1. Communications – Postmortem component
C-1a. Email/letter to those interested in biorepository
This will be a letter mailed to participants who expressed an interest in taking part in the postmortem portion of the biorepository. Because all participants from the pilot study who took part in the postmortem donation also did the in-home biospecimen collection, both are covered in the letter. A copy both fact sheets, HIPAA authorization form, Family authorization form, and consent form(s) will be enclosed with this letter.
[Date]
Name
Address
City, State Zip
Dear [Participant Name]:
The Agency for Toxic Substances and Disease Registry (ATSDR) has launched the National ALS Biorepository (Biorepository). The Biorepository is a new part of the National ALS Registry. They have contracted with McKing Consulting (McKing) to manage the Biorepository. McKing would like to thank you for your interest in the Biorepository. The Biorepository will collect, store and distribute specimens from individuals with ALS. You are free to join or not. Your decision will have no impact on your eligibility to take part in the National ALS Registry or any future studies.
We are asking people who are enrolled in the National ALS Registry to donate biological specimens. This part of the Biorepository, may collect blood, urine, hair and nail specimens at your home. If you choose to take part in this part of the Biorepository, you will be asked to answer a few questions and to provide blood, urine, hair and nail specimens.
We are enclosing a fact sheet that explains the biospecimen part of the Biorepository. It also has answers to some frequently asked questions. We have enclosed a copy of the consent form for the Biorepository. Signing this form indicates that you agree to take part in the Biorepository. We will call you to review the consent form and answer any questions. If any part of this form is not clear to you, be sure to ask questions. Do not sign until you get answers to all of your questions. Once you have reviewed the consent form and agree to participate in the biospecimen part of the Biorepository, we will set up an appointment for a phlebotomist to come to your home and collect specimens.
We are asking individuals who are enrolled in the National ALS Registry to donate specimens postmortem. This part of the Biorepository will collect the brain, spinal cord, cerebral spinal fluid (CSF), muscle, bone, and a skin sample.
We are enclosing a fact sheet that explains the postmortem part of the Biorepository. It also has answers to some frequently asked questions. Enclosed you will find a copy of the Fact Sheet, consent forms, HIPAA authorization form, and family authorization form for the Biorepository. The HIPAA Authorization will allow your physician to discuss your health status with us as part of the eligibility screening for the Biorepository. If we determine that you are eligible to participate in this biorepository, we will schedule a phone appointment with you and a family member to review the consent and authorization forms, answer any questions, and discuss End of Life planning to complete your donation plan. If any part of these forms or processes is not clear to you, be sure to ask questions. When you sign the consent form, it will indicate that you agree to take part in the Biorepository. Do not sign until you get answers to all of your questions. Once you have reviewed the consent form and agree to take part in the postmortem part of the Biorepository, we will finalize all arrangements necessary in order for you to participate in the Biorepository.
Your specimens will be saved and banked for future ALS research. We will store your specimens with some data about you, such as your age, race, and sex. We will not put your name on the specimen. We do not know how long we will keep these specimens. All information you provide will be kept private to the extent allowed by law. To protect your privacy your name will not appear with any of your information. A summary of the results of this specimen collection will be posted on the National ALS Registry website. Individuals will not be identified. Taking part in the Biorepository may help us learn more about ALS. Although the Biorepository will not benefit you directly it may be of future benefit to others.
If you have any questions or want more information, please contact the Biorepository Coordinator, Laurie Wagner by email at lwagner@secure.mcking.com or by phone at 1-855-874-6912.
Thank you for supporting the National ALS Biorepository. We look forward to hearing from you soon.
Sincerely,
Wendy E. Kaye, PhD Senior Scientist, National ALS Biorepository
Enclosures
Reading level 10.6 (changing biorepository to study 8.6)
C-1b. Phone script to begin eligibility screening
This is a phone script for making calls to individuals that received an information packet. The purpose of this call is to screen potential participant to determine if they are eligible for the biorepository. After this call the HIPAA authorization will be signed and returned to participant coordinator.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF POTENTIAL PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: _______________________________________
Time: ___________________________________________
If person on phone asks for your contact information leave your name and phone number only
Thank them and end call
IF POTENTIAL PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository
Ask if he/she received the information packet
Ask if he/she has any questions
State the purpose of the biorepository
Inform the potential participant of the specimens being collected
Ask potential participant if they want to participate in the biorepository
IF POTENTIAL PARTICIPANT DOES NOT WANT TO PARTICIPATE:
Give them the toll free phone number
Thank them and end call
IF POTENTIAL PARTICIPANT IS UNSURE IF THEY WANT TO PARTICIPATE:
Inform potential participant that you will call back in 1 week to see if they have made a decision
Verify contact information and best time to call next week
Thank them and end call
IF POTENTIAL PARTICIPANT DOES WANT TO PARTICIPATE:
Thank participant for agreeing to participate
Inform participant that they will need to return their HIPAA authorization form and then we will contact their physician
Once we have spoken with their physician’s office, we will call the participant back
Thank participant and end call
C-1c. Phone script to provide eligibility screening update
This is a phone script for receiving incoming calls from potential participants to provide them with an update on the status of their screening to determine whether or not they can take part in the biorepository.
INTRODUCTION:
Answer phone, state your name and that you are with the National ALS Biorepository
Ask potential participant how you can help them
IF POTENTIAL PARTICIPANT WANTS AN UPDATE ON THE STATUS OF THEIR SCREENING
Look in database to determine whether or not they have been approved to take part in biorepository;
If not approved, tell potential participant that they are not eligible to take part in the postmortem potion
Ask if they would like to take part in the biospecimen portion
Discuss the biospecimen portion
Verify their contact information
Thank them and end call
If pending, give update on status (waiting on response from physician, etc.)
Tell potential participant that we will call them when we find out whether or not they are eligible to take part.
Verify their contact information
Thank them and end call
If approved, tell potential participant they are eligible to participate
WHAT’S NEXT (for approved participants):
Tell participant that you will now be scheduling their appointment for the consent appointment
Inform participant that a family member needs to be present for this appointment with them
Schedule appointment
Day/Date: ___________________________________
Time (am/pm):________________________________
CONFIRM PARTICIPANT CONTACT INFORMATION:
Read participant the contact information that you have on file and make any necessary corrections; (name, address, phone, email)
Ask if participant prefers US mail or email communication.
WHAT’S NEXT:
Ask participant if there is anyone in the household that you can speak to about the biorepository if they are not available to come to the phone
If no,
Make note in database
If yes,
Document name and relationship to participant
Name of person: ________________________________________
Relationship: ___________________________________________
IN CLOSING:
Confirm date and time of the consent appointment
Ask if participant has any questions
Ask participant to review consent form prior to the consent appointment
Provide your name
Thank them and end call
C-1d. Phone script to inform potential participants that physician is not responding
This is a phone script for making calls to potential participants to notify them that their physician is not responding to the patient health status request.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF POTENTIAL PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: ________________________________
Time: ___________________________________
If person on phone asks for your contact information leave your name and phone number only
Thank them and end call
IF POTENTIAL PARTICIPANT IS AVAILABLE:
Introduce yourself and let the potential participant know that you are calling on behalf of the National ALS Biorepository
Inform potential participant that their physician is not responding to our request for information
Tell potential participant that they cannot take part in the postmortem portion of the biorepository without us getting information from their physicians
Ask if he/she is comfortable talking to his/her physician to encourage him/her to provide the information needed.
Thank them and tell them to have the physician call you directly
Tell potential participant that you will contact them when you get information from their physicians
Ask if they have any questions
Remind participant of your name
Thank them and end call
C-1e. Phone script to notify ineligible participants
This is a phone script for making calls to potential participants that are not eligible for the postmortem portion.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF POTENTIAL PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: _______________________________________
Time: ___________________________________________
If person on phone asks for your contact information leave your name and phone number only
Thank them and end call
IF POTENTIAL PARTICIPANT IS AVAILABLE:
Introduce yourself and let the potential participant know that you are calling on behalf of the National ALS Biorepository
Tell potential participant that they are not eligible to participate in the postmortem part of the biorepository because;
Physician was not available and has not returned our call
They did not meet the eligibility criteria
They are already committed to another postmortem study
Other__________________________________________
We will provide these potential participants information on other biobanks in which they might participate
VERIFY IF PARTICIPANT IS INTERESTED IN THE BIOSPECIMEN PART
Continue biospecimen portion of the biorepository
Thank them and end call
C-1f. Phone script to schedule consent appointment
This is a phone script for making calls to potential participant to inform them that they are eligible to participate in the postmortem part of the biorepository and to schedule a consent appointment.
INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF POTENTIAL PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: _______________________________________
Time: ___________________________________________
If person on phone asks for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository
Inform participant that they are eligible to participate
Tell participant that you will now be scheduling their appointment for the consent appointment
Inform the participant that a family member should also be present at this appointment
Schedule consent appointment
Day/Date: ___________________________________
Time (am/pm):________________________________
CONFIRM PARTICIPANT CONTACT INFORMATION:
Read participant the contact information that you have on file and make any necessary corrections; (name, address, phone, email)
Ask participant if they are able to sign their name
If no,
Inform participant that after the consent form is reviewed, questions are answered and they agree to take part, a family member will need to sign the consent form as a witness.
If yes,
Inform participant that after the consent form is reviewed, questions are answered and they agree to take part, they will then be asked to sign the consent form.
WHAT’S NEXT:
Ask participant if there is anyone in the household that you can speak to about the biorepository if they are not available to come to the phone
If no,
Make note in database
If yes,
Document name and relationship to participant
Name of person: ________________________________________
Relationship: ___________________________________________
IN CLOSING:
Confirm date and time of the consent form appointment
Ask if participant has any questions
Ask participant to review consent form prior to consent appointment
Remind participant that a family member needs to be present
Remind participant of your name
Thank them and end call
C-1g. Email/letter to confirm appointment
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent within 1 week prior to the consent appointment. The purpose of the letter is to confirm the appointment.
[Date]
Name
Address
City, State, Zip Code
Dear [Participant Name]:
This letter is to confirm your call for Participant Coordinator, [name] to go over the National ALS Biorepository Consent form. He/She will also answer any questions that you and your family may have.
Your consent call is scheduled for (Day/Date): _________________ at (Time): __________________. [Add the following sentences if you are using the conference line: Please call our conference line at 1-800-xxx-xxxx. The passcode is xxxxxx#. We will send a copy of this letter to your family member who will be on the call with you.]
He/she will also ask your family member to sign the family authorization form. Please make sure your family member is able to do so. He/she will also review end of life planning to complete your donation plan.
If you have any questions before your call feel free to contact me or your Participant Coordinator, [name] by phone at 1-855-874-6912 or by email at alsbiorepository@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Laurie Wagner, MPH
Coordinator, National ALS Biorepository
cc: Family member
Reading level 10.0 (without study name 8.9)
C-1h. – Phone script to obtain consent
The purpose of this call is to consent the individual through a thorough review of the consent form. After the individual has provided consent, make an appointment for biospecimen collection.
INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with potential participant
IF PARTICIPANT IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
If person asks on phone for your contact information leave your name and phone number only
Thank them and end call
IF PARTICIPANT IS AVAILABLE:
Introduce yourself and let the participant know that you are calling on behalf of the National ALS Biorepository
Inform participant that you are calling to review the consent form
Ask participant if he/she and a family member have had a chance to review the consent form and have a copy available at this time
a) If participant does not have a copy of the consent form available:
Offer to send or email another copy and reschedule a time to call back (depending on their mode of contact.
RECORD TIME FOR NEXT CONTACT:
Day/Date: ____________________________
Time: ________________________________
Remind participant to review consent form and to have a copy available for the next call
Thank them and end call
If participant does have a copy of consent form but has not reviewed it:
Allow participant time to review consent form while you wait on the phone
Ask if participant wants to continue to participate
If participant does have a copy available and has reviewed consent form:
Ask if participant wants to continue to participate
IF PARTICIPANT DOES NOT WANT TO CONTINUE TO PARTICIPATE:
Remind participant of your name
Thank them and end call
IF PARTICIPANT DOES WANT TO CONTINUE TO PARTICIPATE:
Review each section of the consent form
Ask participant if they have any questions at the end of each section
Ask participant if they still want to participate
If no,
Remind participant of your name
Thank them and end call
If yes,
Ask participant or witness to sign the consent form
Ask family member to sign the family authorization form
Inform participant that the signed copy of all consent and authorization forms should be mailed back in the pre-stamped envelope sent with the information packet
CONFIRM PARTICIPANT CONTACT INFORMATION:
Review the contact information that you have on file and make any corrections necessary; (name, address, phone, email etc.)
SCHEDULE BIOSPECIMEN APPONTMENT DATE AND TIME (if applicable):
WHAT’S NEXT:
Schedule appointment for in-home specimen collection
Inform participant that a box containing the collection kit should arrive at their home
Inform participant that the phlebotomist will call them 1 day before their appointment as a reminder
Ask participant if there is anyone in the household that you can speak to about the biorepository if he/she are not available to come to the phone
If no,
Make note in database not to speak with anyone else in the home
If yes,
Document name and relationship to participant
IN CLOSING:
Ask participant if they have any questions
Remind participant of your name
Thank them and end call
C-1i. Email/letter to those who have not agreed to participate
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent to the participant following completion of the consent appointment to thank the participant for considering participation in the biorepository (these potential participants did not sign consent form and have not yet agreed to participate)
[Date]
Name
Address
City, State, Zip Code
Dear [Participant Name]:
Thank you for speaking with the Participant Coordinator, [name], to discuss taking part in the postmortem collection of the National ALS Biorepository. This Biorepository is being done to collect, store, and distribute specimens from individuals with ALS. You were not sure if you wanted to take part in the biorepository when we spoke and wanted to think about it. If we do not hear from you within the next two weeks, we will contact you to see if you have decided.
Please contact us if you have any questions. The Biorepository Coordinator, Laurie Wagner, or your Participant Coordinator, [name], can be reached by phone at 1-855-874-6912. You can reach us by email at alsbiorepository@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Reading level 12.1 (changing biorepository to study 9.2)
C-1j. Phone script to contact those who have not agreed to participate
This phone script will be used when the participant does not sign the consent form during the appointment and they call to tell McKing their decision or McKing calls them to follow up.
OUTGOING CALL INTRODUCTION:
Ask for potential participant, introduce yourself and say that you are following up with potential participant
IF POTENTIAL PARTICIPANT OR APPROVED FAMILY MEMBER IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: _____________________________
Time: ________________________________
If person asks for your information, then leave your name and phone number only
Thank them and end call
INCOMING CALL INTRODUCTION OR IF POTENTIAL PARTICIPANT IS AVAILABLE:
Introduce yourself and let the potential participant know that you are with the National ALS Biorepository
Determine whether or not participant wants to participate and has signed the consent form.
IF POTENTIAL PARTICIPANT DOES NOT WANT TO PARTICIPATE OR IS NOT INTERESTED:
Give them the toll free phone number
Thank them and end call
IF POTENTIAL PARTICIPANT IS STILL UNSURE IF THEY WANT TO PARTICIPATE AT THIS TIME:
Inform potential participant that you will call back in 1 week to see if they have made a decision
Obtain or verify their phone number
Contact Phone: ___________________________
Best times to call (am/pm): _________________
Thank them and end call
IF POTENTIAL PARTICIPANT DOES WANT TO PARTICIPATE AND HAS SIGNED THE CONSENT FORM:
Express gratitude for their interest
Ask participant to mail their consent form to McKing in the self-addressed stamped envelope
WHAT’S NEXT:
Let the potential participant know that we will follow up with them when we receive
signed Consent Form
signed Family Authorization Form
information needed to finalize their Donation Plan
If applicable, schedule biospecimen collection appointment
IN CLOSING:
Ask if they have any questions
Provide your name
Thank them and end call
C-1k. Email/letter thank you for agreeing to participate
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent to the participant following completion of the consent appointment to thank them for agreeing to participate.
[Date]
Name
Address
City, State, Zip Code
Dear [Participant Name]:
Thank you for agreeing to take part in after death tissue collection part of the National ALS Biorepository. This Biorepository is being done to collect, store, and distribute specimens from individuals with ALS. A copy of your signed consent forms and HIPAA authorization form, it is enclosed.
Many studies into the causes, progression, and prevention of ALS need tissue samples to do the research. Your tissues will be available to ALS Researchers. Your generous donation will help ALS research in the future. Donated tissues can lead to research that can improve clinical care for ALS patients.
As we discussed when you agreed to be in the biorepository, we will make a donation plan specific for you. The plan makes sure that we and your family have all the information to ensure a smooth donation process. Within the next few weeks you will receive a copy of your donation plan.
Please contact us if you have any questions or you are unable to take part in the biorepository for any reason. The Biorepository Coordinator, Laurie Wagner, or Participant Coordinator, [name], can be reached by phone at 1-855-874-6912. You can also reach us by email at alsbiorepository@secure.mcking.com. Thank you for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Reading level 11.1 (changing biorepository to 9.1)
C-1l. Email/letter thank you and Donation Plan
This will be sent as an email or letter depending on preference of communication indicated by the participant when consent was obtained. This letter will be sent to the participant following completion of the consent appointment and will include the final donation plan.
[Date]
Name
Address
City, State, Zip Code
Dear [Participant Name]:
Thank you for agreeing to take part in the National ALS Biorepository. Your generous donation will help future ALS research. The Participant Coordinator has made a plan for your donation. The plan makes sure that we and your family have all the information to ensure a smooth donation process. Your donation plan is attached to this letter.
Please contact us if you have any questions, or if any changes need to be made to your donation plan. The Biorepository Coordinator, Laurie Wagner, or your Participant Coordinator, [name] can be reached by phone at 1-855-874-6912. You can also reach us by email at alsbiorepository@secure.mcking.com. Once again, we would like to thank you for your significant donation and for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Donation Plan
|
|
|
{Note delete this item if donation took place at the funeral home being used by the participant}
|
Reading level 11.4 (changing biorepository to study 9.5)
C-1m. Phone script to get health status updates
This is a phone script for making calls to participant to get a status update of the participant’s health. (There will be several calls made from this script)
OUTGOING CALL INTRODUCTION:
Ask for participant, introduce yourself and say that you are following up with potential participant
If participant is not available, ask for family member that participant has approved us to speak with
IF POTENTIAL PARTICIPANT OR APPROVED FAMILY MEMBER IS NOT AVAILABLE:
Ask when would be a good time to contact (him/her)?
RECORD TIME FOR NEXT CONTACT
Day/Date: _____________________________
Time: ________________________________
If person asks for your information, then leave your name and phone number only Thank them and end call
IF POTENTIAL PARTICIPANT OR APPROVED FAMILY MEMBER IS AVAILABLE
Introduce yourself and let the potential participant know that you are calling on behalf of the National ALS Biorepository
Remind them of the purpose of the biorepository
Ask how their last appointment went with their health provider
On every other call (every 6 months), remind them to log onto the National ALS Registry and complete an update of the ALS Functional Rating Score questionnaire
WHAT’S NEXT:
Let the potential participant know that we will call them in 3 months
Verify contact information and best times to call (am/pm)
IN CLOSING:
Ask if they have any questions
Provide your name
Thank them and end call
C-1n. Email/letter thank you for family
This will be sent as a letter to the participant's family. This letter will be sent following the postmortem donation to recognize the donation and to thank the family members for their support.
[Date]
Dear [Family Member Name]:
On behalf of the National ALS Biorepository Team, we would like to extend our condolences on the loss of your [family member]. Dealing with the death of a loved one is never easy. We would like to thank you for honoring your [family member]’s wish to make a tissue donation.
This Biorepository is being done to collect, store, and distribute specimens from individuals with ALS. We will summarize and post updates about the Biorepository on the National ALS Registry website for you and other interested persons to view. Many studies into the causes, progression, and prevention of ALS need tissue samples to do the research. Tissues donated by your [family member] will be available to ALS Researchers. Your [family member’s] generous donation will help ALS research in the future. Donated tissues can lead to research that can improve clinical care for ALS patients.
We realize this is a difficult time and hope that your [family member]’s contribution brings you comfort now and in the days ahead. In a couple of months, the neuropathologist will have completed his review. Please let us know if you would like us to send you a copy of the report. After you have reviewed the report, you can call us to arrange a call with the neuropathologist if you would like to discuss it or have any questions.
If you have any questions, please contact Laurie Wagner, the Biorepository Coordinator, by phone at 1-855-874-6912. You can also reach her by email at lwagner@secure.mcking.com. Thank you again for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Reading level 11.2 (changing biorepository to study 9.7)
C-1o. Email/letter thank you for family where donation failed
This will be sent as a letter to the participant's family. This letter will be sent following the postmortem donation to recognize the donation attempt and to thank the family members for their support.
[Date]
Dear [Family Member Name]:
On behalf of the National ALS Biorepository Team, we would like to extend our condolences on the loss of your [family member]. Dealing with the death of a loved one is never easy. We would like to thank you for honoring your [family member]’s wish to make a tissue donation.
This Biorepository is being done to collect, store, and distribute specimens from individuals with ALS. We will summarize and post updates about the Biorepository on the National ALS Registry website for you and other interested persons to view. Many studies into the causes, progression, and prevention of ALS need tissue samples to do the research. As you are aware, we were unable to recover the postmortem tissues that your [family member] was willing to donate. Although we are all disappointed that we were unable to recover the postmortem tissues, please know that the other specimens (blood, hair, nails or saliva) donated will be available to ALS Researchers. Your [family member’s] generous donation will help ALS research in the future. Donated tissues can lead to research that can improve clinical care for ALS patients.
We realize this is a difficult time and hope that your [family member]’s contribution brings you comfort now and in the days ahead. If you have any questions, please contact Laurie Wagner, the Biorepository Coordinator, by phone at 1-855-874-6912. You can also reach her by email at lwagner@secure.mcking.com. Thank you again for your support of the National ALS Biorepository.
Sincerely,
Wendy E. Kaye, PhD
Senior Scientist, National ALS Biorepository
Reading level 11.6 (changing biorepository to study name 10.4)
Appendix C-2. Other participant materials – Postmortem component
C-2a. Fact Sheet
This will serve as a FAQ document. It will be included in the packet of information mailed to selected registry enrollees that have indicated interest in participating in the postmortem part of the biorepository. It will also be emailed or mailed to any enrollees that ask for more information about the biorepository.
National
Amyotrophic Lateral Sclerosis (ALS) Registry Biorepository Fact
Sheet: Postmortem
|
The National ALS Biorepository (Biorepository) was created by the Agency for Toxic Substances and Disease Registry (ATSDR) to collect, store, and share samples from participants in the National ALS Registry. |
What is a Biorepository? |
A biorepository is a facility that collects and stores samples of biological material. This could include blood, urine, tissue, cells, DNA, and proteins. Some medical information may also be stored along with a written consent form. These samples may be used for future research. |
Why is this Biorepository important? |
The tissues in this biorepository will add to the Registry’s data about ALS. It will also add to the total numbers of tissues available for research on ALS. The National ALS Biorepository will differ from others already in existence because it will include ALS patients from the entire country. In addition, it will not be limited to those with specific exposures or clinical findings. |
Who can take part in the Biorepository? |
People that are enrolled in the National ALS Registry that meet the requirements of the biorepository. We will contact your physicians for more information about your disease to see if you can take part. |
What tissues will be collected in this Biorepository? |
The tissues being collected after death include the brain, spinal cord, cerebral spinal fluid (CSF), muscle, and bone. Any future research will have to be approved by an ethics committee. |
|
|
|
|
Where will the tissues be collected? |
McKing will transport your body to a local collection facility and then to the funeral home of your choice within your local area. |
Is there any risk to me? |
The only risk to you is the possible loss of confidentiality though we will protect this as much as possible. The funeral arrangements may be postponed but our goal is to return your body to the funeral home within three days. The health care workers who take your tissues and organs will treat your body like they would in surgery so that there are few marks and your body is not disfigured. |
Is there any benefit to me? |
There is no direct benefit to you. The Biorepository collects, processes and stores postmortem specimens. However, with further research, your samples may help us better understand ALS in the future. |
Will the information I tell you be kept private? |
Yes. Just like when you talk to your doctor, everything you tell us will be kept private to the extent allowed by law. Any information with your name on it will be kept in a locked area. Only authorized employees will be able to look at this information. Results will be posted on the National ALS Registry website. Individuals will not be identified. |
What will happen after I provide specimens? |
Results of the Biorepository will be summarized and posted on the National ALS Registry website for all participants and other interested persons to view. Individuals will not be identified. ATSDR will keep your specimens for future ALS research. |
How do I take part in the Biorepository? |
First, we need to determine your eligibility to take part by speaking to your doctor about your health status and disease. If you are eligible to take part, our staff will schedule a consent appointment with you and a family member. |
What do I need to do in order to take part in the Biorepository? |
Give McKing permission to speak with your physician. Have a family member available for the consent appointment, who will also need to agree to that you will take part in the Biorepository. Be prepared to make plans for your final arrangements. Agree to provide an update of your health status to McKing on a regular basis. Have a family member agree to notify McKing of your passing within 6-8 hours. |
How long will the consent appointment take? |
A trained professional will call your home and speak with you and a family member to review the consent form and answer all questions you and your family may have about the Biorepository. The review of the consent form and answering questions should take about one hour. |
What if my family does not agree with my wishes to donate tissues after my death? |
It is important to make the decision to provide tissues after your death with your family. We will try to honor your request but if your family objects to donating tissues after your death, we will not be able to include you in the Biorepository. |
Is there any cost to me to donate tissues? |
No. There is no cost to you to donate tissues to Biorepository. |
Do I have to participate in the Biorepository? |
No. Taking part in the Biorepository is completely voluntary. You may also refuse to answer any question for any reason and you may choose to leave the Biorepository at any time even after signing the consent form. There is no penalty for leaving the Biorepository. Your decision will have no impact on your medical care, other services provided by your neurologist, or your participation in the National ALS Registry. |
Who is managing the Biorepository? |
This Biorepository is being funded by ATSDR. ATSDR is a federal public health agency located in Atlanta, Georgia. McKing Consulting Corporation (McKing) has been awarded a contract to collect and distribute specimens for the Biorepository. |
For more information about the Biorepository, contact Laurie Wagner, MPH, Biorepository Coordinator Toll-free: 1-855-874-6912 Email: lwagner@secure.mcking.com |
|
Reading level 9.0 (changing biorepository to study 7.4) |
|
C-2b. HIPAA Authorization Form
This document will be mailed to participant and signed giving McKing permission to contact their physician in order to determine potential participant's eligibility for the biorepository.
The National ALS Biorepository
Health Insurance Portability and Accountability Act (HIPAA)
Authorization for Use and Disclosure of Health Information
Page
1 of 3
What health information do you want? We want to contact your physician to get information on your disease to determine if you will be able to take part in this biorepository.
How will you protect this health information after I release it to this biorepository? The information that ATSDR and McKing get from the physician is not subject to HIPAA protection. However, we’ll make every effort to keep any information obtained private to the extent allowed by law. We don’t plan to release personal information to anyone except for the research staff working on this biorepository. We’ll store the information in a secure location. We won’t use names in any reports.
Do I have to sign this form? You can refuse to sign this form. If you don’t sign it, there will be no penalty, and you won’t lose any benefits. However, without your signature, ATSDR and McKing will not be able to get the medical information needed to help us learn more about your health. You will not be able to donate tissue to the National ALS Biorepository after your death.
How long does my permission last? This permission to speak with your physician lasts until we finish this biorepository.
Page
1 of 2
I have read this form and give my permission for ATSDR and McKing to obtain medical information about my medical history and health status. This form (or a photocopy of this form) is my authorization for ATSDR and McKing to ask my physician for this information.
NAME OF POTENTIAL PARTICIPANT (print): _______________________________________________

Please sign here SIGNATURE OF POTENTIAL PARTICIPANT: _______________________________________________
SIGNATURE OF LEGALLY AUTHORIZED REPRESENTATIVE_________________________________________
TODAY’S DATE _________________________________________
|
Please provide the following information about the physician who can provide information about your current health status. This will most likely be your neurologist. Print clearly using upper case block letters (e.g., JOHN DOE).
Provider’s Name : __________________________________________________________________
Contact Name: (ex. Nurse, Medical Assistant)_____________________________________________
Provider’s Phone: ___(_______)_________________________________________________________ Area Code
|
Thank you for completing this form.
Please mail it back to us in the enclosed postage-paid envelope.
Reading level 9.0 (changing biorepository to study 8.3)
Page
2 of 2
C-2c. Eligibility Checklist
This document will guide the conversation that biorepository staff has with the donor’s physician to assess the eligibility of the PALS in order to determine if they can participate in the Biorepository study.
PALS interested in participating in the National ALS Biorepository must meet the following criteria:
|
Person does not plan to go on the respirator |
|
Person does not have dementia |
|
Person is in late stage of disease or at a certain progression |
|
Physician doesn’t know of any patient or family member issues/concerns that would make him/her a bad candidate for the biorepository |
|
Anticipated lifespan of patient |
|
In physician’s opinion, participant is good candidate for this biorepository |
C-2d. Family Authorization Form
This form will be included in the information packet mailed to selected registry enrollees that have indicated interest in participating in the biorepository. It will be signed by a designated family member after the research coordinator conducts the consent call.
Family Authorization Form
Donor’s Name
Your family member (listed above) agreed to take part in the postmortem donation of the National ALS Biorepository. This biorepository is being done to learn more about collecting and storing specimens from persons with ALS. We are asking people who have joined the National ALS Registry to donate tissues after death. This biorepository will collect the brain, spinal cord, cerebral spinal fluid (CSF), and a small piece of muscle, and bone. The Agency for Toxic Substances and Disease Registry awarded McKing Consulting Corporation (McKing) a contract to conduct this biorepository.
By signing this form, you recognize your family member’s wishes to take part in this biorepository. You agree to honor your family member’s wishes by allowing McKing to collect and store the donation. The donation consists of your family member’s brain, spinal cord, CSF, and a small piece of muscle, bone, and skin.
______________________________________________________________________________
Signature of Family Member Date
______________________________________________________________________________
Print Name Relationship
______________________________________________________________________________
Phone number
______________________________________________________________________________
FOR RESEARCH COORDINATORS USE ONLY: Consent must be confirmed by family member following the death of the donor.
Confirmed via: _______ telephone _______fax _______in-person ________other
______________________________________________________________________________
Confirmed by (Family Member) Relationship Date
Reading level 11.9 (changing biorepository to study 9.8)
National ALS Biorepository Consent Form (Postmortem)
Why am I being asked?
You are being asked to take part in the biorepository because you are enrolled in the National ALS Registry and have told us you are interested in learning more about the biorepository. We ask that you read this form and ask any questions you may have before agreeing to be in the biorepository. McKing is the company that was awarded a contract by the Agency for Toxic Substances and Disease Registry (ATSDR) to manage the biorepository. The biorepository Senior Scientist is Dr. Wendy E. Kaye.
Taking part in this biorepository is voluntary. You should feel free to ask the researchers any questions you may have. Your decision whether or not to take part will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
What is the purpose of this biorepository?
Page
1 of 5
Approximately 40 people will take part in the collection of tissues after death part of the biorepository.
What are the procedures involved?
You will be asked to set up a call that includes a family member for us to discuss the biorepository. After you have consented to take part, the coordinator will work with you or your family to create a donation plan. This plan will include all logistics for donating tissues after your death. We will also ask one of your family members to sign an authorization form because it is important that your family agree with this donation after your death.
The tissues being collected include the brain, spinal cord, cerebral spinal fluid (CSF), muscle, and bone.
Making arrangements for the tissue donation prior to death
The National Disease Research Interchange (NDRI), a group dedicated to collecting specimens for research, will work with us to obtain your donation. Someone from NDRI will call you if you agree to take part and answer any questions you may have about the donation. They will find a person and facility where tissue removal will happen. This will happen after you agree to be a tissue donor and before your death. This facility will be as close to your home as possible. We will take care of the transportation of your body until you are at the funeral home selected by you and your family.
Tissue donation after death
Upon your death, the following steps will be taken to collect your donated tissue:
We will ask your selected family member to verbally agree to go forward with the donation. The donation cannot occur if he/she does not agree.
We will have your body transported to the facility where the tissue collection will happen.
We will arrange for the tissue collection process to happen within 24-36 hours of death. The collection will take about two to three hours to complete.
Your donated tissue will be express shipped by a special courier to the approved storage facility.
Your body will be returned to your family’s funeral home or to whatever facility that your family has arranged within three days of your death.
Page
2 of 5
We will make a good faith effort to collect your tissues. However, there may be things beyond our control that may prevent collection of your samples.
Tissue Banking
The purpose of this biorepository is to collect specimens for future research related to ALS. Your specimens will be stored with a number. No private information will be on the specimen container.
Researchers can ask to use specimens from the biorepository for research related to ALS. We do not know what types of research will be done. These studies will likely examine the DNA and RNA (genetic material) and other material from cells in the tissues. Researchers must provide a detailed plan of the study. This plan must be approved by ATSDR and the ethics committees at the researchers’ institutions. Your specimens along with others will be provided to approved researchers. These tissues will not have your name on them. The specimens will include information about you such as your age or city where you lived. They may also request other data you gave the National ALS Registry. We will limit the amount of information about you to reduce the chances that you will be identified.
Results of research using these tissues will be available on the National ALS Registry website.
What are some of the risks and discomforts that may happen to people who are in this biorepository?
There is a slight risk that someone could get access to the data we have stored about you. If information about you does leak out, ATSDR will not be able to guarantee that it will be protected.
There may be unforeseen problems that may delay the delivery of your body to the funeral home. We do not think this will happen but we cannot be sure.
All tissue samples will be collected at the time of your death. This will be a sensitive time for your family members. Some members may not agree with you and your selected family member’s choice to allow the donation. This may cause some discomfort among family members.
Future research may show that you and/or your family are at increased risk of developing a disease or condition. We will not provide this information to your family members.
If future research results show that certain groups (for example, racial, ethnic, or men/women) have genes that are associated with increased risk of a disease, then this information could make it harder for your family to obtain employment, adopt children, or get medical and life insurance. There are laws against the misuse of genetic information, but they may not give full protection. We believe the chance these things will happen is very small.
Rare, unknown, or unanticipated risks also may occur.
ATSDR will do all it can to protect your privacy and confidentiality (see below). Therefore, these risks of discrimination are very small. But the risk may grow in the future if people come up with new ways of tracing information.
Page
3 of 5
Are there benefits to taking part in this biorepository?
You will not directly benefit from taking part in the biorepository. It is hoped that knowledge gained from this biorepository may benefit others with ALS in the future.
What other options are there?
You have the option to not to take part in this biorepository.
What about privacy and confidentiality?
If you take part, we will not have access to your private medical records. However, we will have to ask your doctor to share some information about you.
The people who will know that you take part in this biorepository are members of the biorepository team, individuals who may have access to your informed consent document due to their job function with the National ALS Biorepository, and people processing your tissues.
Information that identifies you and the consent form signed by you will be looked at by the ATSDR.
A possible risk of the biorepository is that people outside of the biorepository may find out you are taking part or learn information about you and your health. Your tissues will be stored with a code. Your name will not be on the container.
When the results are published or discussed at meetings, no information will be included that would reveal your identity
Will my family be told biorepository results?
Your family can get the results of the neuropathology review. When the review is complete, the neuropathologist will check with your family to see if they want the report and answer any questions. Your family will not be told of any new findings that come to light during the course of this biorepository. We will not tell you or your family any genetic testing results from research studies. ATSDR will post biorepository results on the National ALS Registry website.
What are the costs for participating in this biorepository?
There is no cost for participating in this biorepository. The costs of transporting your body to and from the facility and collection procedures for this biorepository will be the responsibility of the ATSDR. There will be no cost to you, your family, or estate.
Will I be reimbursed for any of my expenses or paid for my participation in this biorepository?
You will not be reimbursed for any expenses. You will not be paid to take part in this biorepository.
Will my cells, tissues, blood, or other biological materials be used to develop commercial products?
It is possible that a commercial product may be developed from the tissues collected as part of this biorepository. The nature of the research means that your sample is only one of many that will lead to this product and your sample will not have your name on it. You and your family will not profit financially from such a product. You will have no legal rights to any discovery or invention that either directly or indirectly results from the use of your specimens collected, individual information, or information from your samples.
Cells obtained from your body may be used to establish a cell line which may be shared in the future with other researchers and which may be of commercial value. A cell line is one which will grow indefinitely in the laboratory. Cell lines may be useful because of the characteristics of the cells and/or the products they may produce.
Can I withdraw or be removed from the biorepository?
Taking part in this biorepository is voluntary. If you choose not to take part or decide to withdraw your consent and stop taking part, this will not affect your relationship with National ALS Registry or other benefits to which you are otherwise entitled.
You have the right to leave a biorepository at any time without penalty. Your family can also refuse to have your tissues collected after your death. If you withdraw from the biorepository, we will destroy any of your remaining samples. However, we will not be able to remove your samples that have already been used or shared with researchers.
Page
4 of 5
If you have questions, concerns, or complaints, think the biorepository has hurt you, or if you want to withdraw your samples, you can talk to Wendy E. Kaye, Ph.D., Senior Scientist or Laurie Wagner, MPH, Biorepository Coordinator at 1-855-874-6912.
If you want to speak with someone who is not directly involved in this biorepository, or have questions about your rights, or wish to voice questions, concerns or complaints, you may contact the CDC Human Research Protection Office at 1-800-584-8814.
Remember
Taking part in this biorepository is voluntary. Your decision whether or not to participate will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship.
Subjects’ Rights:
I have read the above information. I have discussed this biorepository with the person obtaining consent, been given an opportunity to ask questions, and my questions have been answered to my satisfaction. I agree to the release of personally identifying information about me to the funeral home. My name, address, telephone number and date of birth are needed to provide transportation to the pathology service and my return to the funeral home selected by my family. I agree to take part in this biorepository. I will be given a copy of this signed and dated form.
Signature of Subject Date

If participant is physically unable to sign the consent form, please complete the following
Witness Signature Date
Page
5 of 5
Reading level 9.9 (changing biorepository to study 8.7)
National ALS Biorepository Consent Form Addendum (Postmortem Skin Collection)
Why am I being asked?
You have agreed to take part in the postmortem tissue collection portion of the National ALS Biorepository.
What is the purpose of this collection?
We would like to put some of the cells from your skin in a cell bank so that researchers can use them in the future. We don’t know what kind of future research will be done but the studies will be ALS related.
What are the procedures involved?
Small skin samples (postmortem) will be collected from up to 40 participants in order to isolate primary fibroblasts. If you choose to take part in the skin collection, after death a small skin sample about the size of a quarter will be surgically removed from your back. It will be sent to an ATSDR approved laboratory for cell isolation. The lab performing this part of the specimen processing is Zen-Bio Incorporated.
Where will my skin sample be stored?
After the skin sample is transferred to Zen-Bio the individual skin cells will be separated from the skin tissue. A particular skin cell type called a fibroblast will be grown in the lab to make more of these cells for future ALS research. These cells can be stored at very low temperatures as frozen samples that can be stored forever, if handled properly. These frozen cells can then be thawed and continue to grow in a laboratory to produce more cells for ALS research. Your skin cells will be kept by an ATSDR approved facility.
What about privacy and confidentiality?
Your skin sample will be coded with an ID number so that your name is not on the sample.
Your skin cells may be shared with researchers in the future. These samples will only be identified by ID numbers. The individual cells contain your genetic information in the form of DNA. There is a very small chance that genetic research in the future could give genetic information that could be used to identify you.
Can I withdraw or be removed from the biorepository?
Taking part in this biorepository is voluntary. If you choose not to take part or decide to withdraw your consent and stop taking part, this will not affect your relationship with National ALS Registry or other benefits to which you are otherwise entitled.
You have the right to leave this biorepository at any time without penalty. Your family can also refuse to have your tissues collected after your death.
Remember
Taking part in this biorepository is voluntary. Your decision whether or not to participate will not affect your current or future relations with the National ALS Registry. If you decide to take part, you are free to withdraw at any time without affecting that relationship. Other information about this biorepository can be found on your copy of the consent form you signed earlier.
Subjects’ Rights:
I have read the above information. I have discussed this biorepository with the person obtaining consent, been given an opportunity to ask questions, and my questions have been answered to my satisfaction. I agree to provide a skin specimen. I will be given a copy of this signed and dated form.
Signature of Subject Date

If participant is physically unable to sign the consent form, please complete the following
Witness Signature Date
I, _____________________, witnessed that _________________ was explained the consent form and has agreed to take part in the skin collection. Due to the progression of the disease, the participant is physically unable to sign the consent form.
Reading Level 8.7 (changing biorepository to study 7.9)
Appendix C-4. Postmortem specimen collection procedures
Appendix C-4a. Postmortem collection of brain, spinal cord, and CSF specimens
Brain and Spinal cord. A contracted diener will obtain the whole brain and spinal cord (cervical to lumbar by either anterior or posterior approach), a procedure which usually takes one to two hours. The brain including brain stem and cerebellum weight will be recorded. The length and weight of the spinal cord will be recorded. The recording sheet is included in the appendix. The whole brain and spinal cord will be shipped in plastic bags placed on wet ice contained in a lidded bucket within an insulated specimen shipper. The diener packs the organs according to instructions included in the shipping materials.
CSF. Postmortem cerebrospinal fluid (CSF) will pool at the foramen magnum when the body is supine and the head is slightly lifted. CSF may then be obtained from the foramen magnum by gently lifting the frontal lobes to access with a syringe attached to a large bore needle. This is done prior to freeing the cerebellum and cutting into the transverse sinuses in order to minimize blood contamination. Alternatively, CSF may be obtained from the lateral ventricles by entering through the top of the corpus callosum with the syringe needle. Approximately 10 mL of clear fluid should be obtained and placed into a 15 mL centrifuge tube.
Appendix C-4b. Postmortem collection of muscle and bone specimens
Muscle. During the spinal cord recovery an approximately 2 x 1 x 1 cm piece of lumbar paraspinous muscle should be removed and placed into a formalin-filled vial.
Bone. Bone sampling will depend on the method used by the pathologist for the spinal cord removal (posterior or anterior approach). For the posterior approach, a piece can be removed with the bone saw and/or chisel from the lamina. If an anterior approach consistent with a full autopsy is used for cord removal, then a piece may be removed from the pedicle or the body of the vertebra. Regardless of the approach, a 1 cm cubed sample should be taken from a lumbar vertebra. This sample should be placed into a separate formalin-filled vial. The storage vial and formalin will be lot tested for metals.
Appendix C-4c. Postmortem collection of skin specimens
During the spinal cord recovery an approximately 1 x 1 in piece of full thickness skin (~6cm2) or 6, 8mm skin biopsy punches (3cm2) will be obtained. Skin samples should be placed in a plastic specimen container containing medium and antibiotics provided by Zen-Bio. Dermal fibroblasts will be isolated and cell lines established.
Appendix C-5. Processing of brain, spinal cord, and CSF specimens
Fresh Brain Dissection and Gross Examination
The brain and spinal cord are analyzed for gross lesions. Each is thoroughly photographed, measured and recorded. The brainstem and cerebellum are removed with a transverse cut through the upper midbrain; then the cerebellum is removed from the brainstem. A single coronal cut through both hemispheres is made 2 mm posterior to the mammillary bodies. Both anterior and posterior halves are photographed. The condition of the fornix, corpus callosum, septum pellucidum are evaluated and photographed. The hemispheres are then bisected. The hemisphere with the greatest focal pathology is fixed by immediate immersion in ice cold 4% periodate-lysine-paraformaldehyde (PLP) (pH 7.4). Information collected during the gross pathology on the spinal cord is documented as a free-form written description. It would include things such any abnormalities in the color and size of the spinal cord and spinal nerve roots.
The hemisphere to be frozen is cut coronally in 0.8 cm coronal sections. The posterior surfaces of all sections are photographed. The slabs are flash frozen flat, between two 0.4 cm thick aluminum Teflon-coated plates separated by 0.8 cm aluminum bolts, previously chilled on dry ice. The slabs are bagged, sealed, and labeled with the subject’s autopsy number and the tissue slab number. The corresponding cerebellar hemisphere is cut sagittally into sections and snap frozen. Four 2 cm levels of spinal cord, including multiple sections of cervical, thoracic, lumbar, and sacral cord, are sectioned and snap frozen. The frozen tissue is transferred immediately to a -80 C freezer. The remainder of the spinal cord is fixed by immediate immersion in ice cold 4% periodate-lysine-paraformaldehyde (PLP) (pH 7.4).
Fixed Brain Tissue and Spinal Cord
The fixed brain tissue and spinal cord is fixed in PLP for 2 weeks and kept at 40C. Afterward, the hemisphere is cut coronally, the cerebellum is cut sagittally and the brainstem transversely in 0.5 cm sections. The posterior surfaces of all tissue slabs are photographed. The fixed spinal cord is cut transversely. The blocks for paraffin processing, including multiple sections of cervical, thoracic, lumbar, and sacral spinal cord, are taken (see Appendix Table); the blocks are fixed in 10% formalin for an additional 7 days, and then processed into slides. The remaining tissue is stored in PLP at 40C.
Cerebrospinal Fluid
Upon arrival at the lab CSF is mixed by gently inverting the tube 5 times. The tubes are centrifuged at 1.5 g for 15 minutes at 4 °C. The CSF supernatant is carefully removed with a transfer pipet, leaving about 300 microliters in the bottom, and aliquoted into 1.5 mL microcentrifuge polypropylene tubes (Fisher Scientific, catalog #02-682-557). The pellet is re-suspended with the remaining 300 microliters of CSF and labeled. The CSF supernatant and pellet are stored with the tissue in the brain bank freezers at -80°C.
Neuropathology Data Collection Form
A 38-page neuropathological data collection form is generated on each case detailing the regional gross and microscopic findings. This data collection form incorporates an expanded version of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) protocol, the National Alzheimer’s Coordinating Center (NACC) data collection worksheet and includes a semi-quantitative regional assessment of neurofibrillary tangles (NFT), Lewy Bodies (LB), diffuse and neuritic senile plaques (SP), argyrophilic grains, tau, ubiquitin and a-synuclein positive inclusions, microvascular lesions, as well as age, location and total area involved in all gross vascular lesions. All information is numerically coded to facilitate data entry and retrieval. A final neuropathology report is generated within 3 months of the date of death and may be sent to the donor’s family if requested. It will be noted if the neuropathology exam does not support a diagnosis of ALS. The final pathology report and neuropathological data collection form will be made available to approved users of the biorepository when requested.
Table: Brain Regions Processed into Paraffin Blocks for Histological Examination
Blocks marked with a * are processed into slides, other blocks are kept in reserve.
1. * Olfactory bulb (3)
2.* Midbrain at level of red nucleus (3)
2A. Midbrain at decussation of the superior cerebellar peduncle
3.* Precentral motor and postcentral sensory cortex (Brodmann area (BA) 4, 3, 2, 1)
4.* Inferior parietal cortex (BA 39,40) (3)
5.* Anterior cingulate (BA 24) (2)
5A. Superior frontal (BA 8)
6. Inferior frontal cortex (BA 10,11,12)
7.* Middle frontal cortex (BA 8,9) at level of CAP (4)
8.* Caudate nucleus, putamen, and nucleus accumbens (CAP)
9. Anterior temporal (BA 38)
10.* Superior temporal (BA 20, 21,22)
11.* Amygdala, with entorhinal cortex (BA 28) (3)
12.* Globus pallidus, putamen with claustrum, insula and substantia innominata (2)
13. Anterior hippocampus
14.* Hippocampal formation at level of lateral geniculate body, tail of caudate (4)
14A* Hippocampus at level of lateral geniculate body, contralateral side
15. Superior temporal posterior (BA 41,42)
16. Thalamus with centromedian, dorsal medial, lateral dorsal and lateral posterior nuclei
16A.* Thalamus with subthalamic nucleus, mammillary body (2)
17. Posterior cingulate (BA23, 31)
18.* Calcarine cortex (BA 17,18) (3)
19. Superior parietal cortex (BA 7b)
20.* Upper pons (level of locus cœruleus) (2)
20A. Lower pons at Vth cranial nerve
21. * Medulla oblongata (including inferior olives)
22A.* Cervical spinal cord
22B.* Thoracic spinal cord
22C. * Lumbar spinal cord
22D. * Lumbar spinal cord
22E. * Sacral spinal cord
23. Cerebellar vermis
24.* Cerebellum with dentate nucleus (2)
25. * BA 19 (4)
Staining methods: Twenty-six blocks are processed into microscopic slides. Adjacent sections are stained with luxol-fast blue, hematoxylin and eosin (LHE), and modified Bielschowsky's silver stain. In addition, immunohistochemistry is performed to detect abnormal accumulations of tau, alpha-synuclein, TDP-43, and ubiquitin using antibodies as follows: AT8 (a mouse monoclonal antibody directed against phosphoserine 202 and phosphothreonine 205 of PHF-tau, Pierce Endogen, Rockford IL, 1:2000), alpha-synuclein (rabbit polyclonal, Chemicon, Temecula, CA, 1:15,000), TDP-43 (a rabbit polyclonal directed against TAR DNA-binding protein 43, Abcam, Cambridge MA, 1:1000, formic acid pretreatment), phospho-TDP-43 ( pS409/410; Cosmo Bio Co., Tokyo, Japan, 1:5000, formic acid pretreatment), and ubiquitin (rabbit polyclonal, Dako , Carpinteria, California 1:2000).
Appendix C-6. Neuropathology data collection form
NEUROPATHOLOGY DATA COLLECTION FORM
|
1. PIN Number: |
2. FHS ID: - |
3. Date Form Completed: -- |
4. Autopsy No.: BVAX A- - |
5. Last Name: _______________________________ (not entered in database) |
6. First Name:_______________________________ |
7. Sex: 1=male, 2=female |
8. Date of Birth: -- |
9. Date of Death: -- |
10. Autopsy Date: -- |
11. PM interval: :hrs-min |
B. TISSUE AVAILABILITY |
|
1. TISSUE AVAILABILTY: 1Yes 2No |
|
2. Infectious tissue |
1Yes 2No |
3. Infectious Disease Comments: |
|
B. TISSUE AVAILABILITY (CONTINUED) |
|||||
4. Fresh |
1Yes 2No |
||||
5. PLP |
1Yes 2No |
||||
6. Formalin |
1Yes 2No |
||||
7. Comments: _________________________________________ ___________________________________________________ |
|||||
8. Frozen Tissue present |
1Right 2Left1N/A |
||||
9. Freezer______________________________________________________________ |
|||||
10.Shelf ________________________________________________________________ |
|||||
11.Comments ___________________________________________________________________ ________________________________________________________________________________ |
|||||
S1 |
|
S2 |
|
||
S3 |
|
S4 |
|
||
S5 |
|
S6 |
|
||
S7 |
|
S8 |
|
||
S9 |
|
S10 |
|
||
S11 |
|
S12 |
|
||
S13 |
|
S14 |
|
||
S15 |
|
S16 |
|
||
S17 |
|
SN |
|
||
Cerebellum |
|
OB |
|
||
14. Paraffin Blocks 1Yes |
Comment: |
||||
1 |
|
2 |
|
2A |
|
3 |
|
4 |
|
4A |
|
5 |
|
5A |
|
6 |
|
7 |
|
7A |
|
8 |
|
9 |
|
10 |
|
10A |
|
11 |
|
11A |
|
12 |
|
13 |
|
13A |
|
14 |
|
14A |
|
15 |
|
16 |
|
16A |
|
17 |
|
18 |
|
19 |
|
20 |
|
20A |
|
20B |
|
21 |
|
21A |
|
22 |
|
23 |
|
24 |
|
25 |
|
25A |
|
26 |
|
26A |
|
B. TISSUE AVAILABILITY (CONTINUED) |
||||
27 |
|
27A |
|
|
28 |
|
28A |
|
|
29 |
|
30 |
|
|
31 |
|
32 |
|
|
15. Did we receive only a single hemisphere? 1Yes 2No |
Comment: |
|||
C. GROSS EXAMINATION |
|||||
1. Brain weight: (in grams) |
|
||||
MENINGES: (scoring 0-4) |
|||||
2. Subdural hemorrhage, chronic |
|
||||
3. Subdural hemorrhage, acute |
|
||||
4. Subarachnoid hemorrhage, chronic |
|
||||
5. Subarachnoid hemorrhage, acute |
|
||||
CEREBRAL BLOOD VESSELS: unremarkable |
|||||
Atherosclerosis of cerebral vessels: Scoring: 0, 1, 2, 3, 4 3= > 50 % occlusion 4= > 90% occlusion |
|||||
|
RIGHT |
LEFT |
|||
6. Internal carotid |
|
|
|||
7. Anterior cerebral |
|
|
|||
8. Middle cerebral |
|
|
|||
9. Posterior cerebral |
|
|
|||
10. Basilar artery |
|
|
|||
C. GROSS EXAMINATION (continued) |
||
11. Vertebral |
|
|
ATROPHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
12. Frontal |
|
|
13. Parietal |
|
|
14. Temporal |
|
|
15. Occipital |
|
|
16. Hippocampal |
|
|
17. Entorhinal |
|
|
18. Amygdala |
|
|
19. Substantia Nigra Pallor |
|
|
20. Locus Ceruleus Pallor |
|
|
D. ACUTE INFARCT(S) (> 1.0 cm in diameter; >2 weeks) |
Total Number: |
|||||||
Total Area (cm)
AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-35 (see codes below) |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATION:
fronto-polar
orbito-frontal
middle-frontal
superior-frontal
anterior cingulated
pre-Rolandic
Rolandic
posterior-medial frontal
Broca (forntal operculum)
temporo-polar
inferior temporal
amygdala/ant hippocampus
hippocampus
middle temporal
superior temporal
auditory area
inferior parietal
superior parietal
angular gyrus (parietal operculum)
calcarine
inferior/lateral occipital
basal forebrain
caudate
putamen
globus pallidus
internal capsule
thalamus
midbrain
pons
medulla
cerebellum
Entire MCA territory (2,3,6,7,8,9,10,14,15,16,17,18,19,21,23,24)
Entire ACA territory (1,2,4,5)
Entire PCA territory (11,13,20)
ACA/MCA territory (1,2,3,4,5,6,7,8,9,10,11,13,14,15,16,17,18,
19,20, 21)
Comments:
______________________________
______________________________ ______________________________
______________________________
______________________________

E. ACUTE LACUNES (<1.0 cm) |
Total Number: |
||||
Total Area (cm) AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-17 (see codes below) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATIONS:
frontal cortex
subcortical white matter, frontal
parietal cortex
subcortical white matter, parietal
temporal cortex
subcortical white matter, temporal
occipital cortex
subcortical white matter, occipital
caudate
putamen
globus pallidus
internal capsule
thalamus

Comments:
______________________________
______________________________
______________________________
______________________________
______________________________
_____________________________
______________________________
______________________________
______________________________
______________________________
midbrainpons
medulla
cerebellum
Comments:
______________________________
______________________________ ______________________________
______________________________
______________________________

F. ACUTE HEMORRHAGES (parenchymal) |
Total Number: |
|||||
Total Area (cm) AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-5 (see codes below) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATIONS:
cerebral cortex
subcortical white matter
deep gray
brainstem
cerebellum
Comments:
___________________________________________
___________________________________________ ___________________________________________ ___________________________________________
___________________________________________ ___________________________________________
___________________________________________ ___________________________________________
____________________________________________
______________________________

G. CHRONIC INFACT(S) (> 1.0 cm in diameter; >2 weeks) |
Total Number: |
|||||||
Total Area (cm)
AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-35 (see codes below) |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATION:
fronto-polar
orbito-frontal
middle-frontal
superior-frontal
anterior cingulated
pre-Rolandic
Rolandic
posterio-medial frontal
Broca (frontal operculum)
temporo-polar
inferior temporal
amygdala/ant hippocampus
hippocampus
middle temporal
superior temporal
auditory area
inferior parietal
superior parietal
angular gyrus (parietal operculum)
calcarine
inferior/lateral occipital
basal forebrain
caudate
putamen
globus pallidus
internal capsule
thalamus
midbrain
pons
medulla
cerebellum
Entire MCA territory (2,3,6,7,8,9,10,14,15,16,17,18,19,21,23,24)
Entire ACA territory (1,2,4,5)
Entire PCA territory (11,13,20)
ACA/MCA territory (1,2,3,4,5,6,7,8,9,10,11,13,14,15,16,17,
18,19,20, 21)
Comments:
__________________________________
__________________________________ __________________________________
__________________________________
__________________________________

H. CHRONIC LACUNES (<1.0 cm) |
Total Number: |
||||
Total Area (cm) AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-17 (see codes below) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATIONS:
frontal cortex
subcortical white matter, frontal
parietal cortex
subcortical white matter, parietal
temporal cortex
subcortical white matter, temporal
occipital cortex
subcortical white matter, occipital
caudate
putamen
globus pallidus
internal capsule
thalamus

Comments:
_______________________________
______________________________________________________________
_______________________________
_______________________________
_______________________________
_______________________________
_________________________________
______________________________
______________________________
midbrainpons
medulla
cerebellum
I. CHRONIC HEMORRHAGES (parenchymal) |
Total Number: |
|||||
Total Area (cm) AP Lateral DV |
R, L or bilat. 1=R, 2=L, 3=bilat |
Locations Scoring 1-5 (see codes below) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOCATIONS:
cerebral cortex
subcortical white matter
deep gray
brainstem
cerebellum
Comments:
_______________________________
_______________________________ _______________________________ _______________________________
_______________________________ _______________________________
_______________________________ _______________________________
____________________________________________
______________________________

J. MICROSCOPIC EXAMINATION: scoring 0-4 |
||||
OLFACTORY BULBS: (AT8) scoring 0-4 |
||||
|
RIGHT |
LEFT |
||
1. NFTs |
|
|
||
2. NTs |
|
|
||
3. Lewy Bodies |
|
|
||
4. Lewy Neurites |
|
|
||
K. Substantia Nigra, Pars Compacta: scoring 0-4 |
|
1. Neuronal Loss |
|
2. Astrocytosis |
|
3. Extraneuronal Pigment |
|
4. Lewy Bodies: Scoring 0-2 |
|
5. Lewy Neurites |
|
6. Pale Bodies: Scoring 0-2 |
|
7. Spheroids |
|
8. NFTs (AT8) |
|
9. Microinfarcts |
|
Pars Reticulata |
|
10. Microinfarcts |
|
11. Neuronal Loss: scoring 0-4 |
|
Cerebral Peduncle |
|
12. Wallerian Degeneration: |
1Yes 2No |
L. ROLANDIC REGION |
||||
|
RIGHT |
LEFT |
||
1. Neuronal Loss: scoring 0-4 |
|
|
||
MICROINFARCTS: number |
||||
Cortex |
||||
|
RIGHT |
LEFT |
||
2. Cavitated |
|
|
||
3. Non-cavitated |
|
|
||
4. Acute |
|
|
||
5. Microhemorrhages |
|
|
||
Subcortical White Matter |
||||
|
RIGHT |
LEFT |
||
6. Cavitated |
|
|
||
7. Non-cavitated |
|
|
||
8. Acute |
|
|
||
9. Microhemorrhages |
|
|
||
10. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
||
11. Spongiform Change: |
|
|
||
Subcortical Deep White Matter: scoring 0-3 |
||||
|
RIGHT |
LEFT |
||
12. Myelin Loss 13. If yes: 1=diffuse, 2=focal |
|
|
||
14. Perivascular rarefaction |
|
|
||
15. Dilation of perivascular spaces |
|
|
||
16. Perivascular macrophages |
|
|
17. Fibrohyalinotic vascular changes |
|
|
M. INFERIOR PARIETAL REGION |
||
AMYLOID ANGIOPATHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
1. Intraparenchymal |
|
|
2. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
Cortex |
||
3. Cavitated |
|
|
4. Non-cavitated |
|
|
5. Acute |
|
|
6. Microhemorrhages |
|
|
Subcortical White Matter |
||
|
RIGHT |
LEFT |
7. Cavitated |
|
|
8. Non-cavitated |
|
|
9. Acute |
|
|
10. Microhemorrhages |
|
|
11. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
12. Spongiform Change: |
|
|
Subcortical Deep White Matter: scoring 0-3 |
||
|
RIGHT |
LEFT |
13. Myelin Loss |
|
|
14. If yes: 1=diffuse, 2=focal |
|
|
15. Perivascular rarefaction |
|
|
16. Dilation of perivascular spaces |
|
|
17. Perivascular macrophages |
|
|
18. Fibrohyalinotic vascular changes |
|
|
|
RIGHT |
LEFT |
19. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
20. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
21. SP (neuritic): scoring 0-4 |
|
|
22. SP (diffuse): scoring 0-4 |
|
|
23. Lewy Bodies: scoring 0-2 |
|
|
Anterior Cingulate |
||
24. Lewy Bodies: scoring 0-2 |
|
|
N. MIDDLE FRONTAL REGION |
||
AMYLOID ANGIOPATHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
1. Intraparenchymal |
|
|
2. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
Cortex |
||
3. Cavitated |
|
|
4. Non-cavitated |
|
|
5. Acute |
|
|
6. Microhemorrhages |
|
|
Subcortical White Matter |
||
|
RIGHT |
LEFT |
7. Cavitated |
|
|
8. Non-cavitated |
|
|
9. Acute |
|
|
10. Microhemorrhages |
|
|
11. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
12. Spongiform Change: |
|
|
Subcortical Deep White Matter: scoring 0-3 |
||
|
RIGHT |
LEFT |
13. Myelin Loss
14. If yes: 1=diffuse, 2=focal |
|
|
15. Perivascular rarefaction |
|
|
16. Dilation of perivascular spaces |
|
|
17. Perivascular macrophages |
|
|
18. Fibrohyalinotic vascular changes |
|
|
|
RIGHT |
LEFT |
19. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
20. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
21. SP (neuritic): scoring 0-4 |
|
|
22. SP (diffuse): scoring 0-4 |
|
|
23. Lewy Bodies: scoring 0-2 |
|
|
O. TEMPORAL REGION |
||
AMYLOID ANGIOPATHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
1. Intraparenchymal |
|
|
2. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
Cortex |
||
3. Cavitated |
|
|
4. Non-cavitated |
|
|
5. Acute |
|
|
6. Microhemorrhages |
|
|
Subcortical White Matter |
||
|
RIGHT |
LEFT |
7. Cavitated |
|
|
8. Non-cavitated |
|
|
9. Acute |
|
|
10. Microhemorrhages |
|
|
11. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
12. Spongiform Change: |
|
|
Subcortical Deep White Matter: scoring 0-3 |
||
|
RIGHT |
LEFT |
13. Myelin Loss |
|
|
14. If yes: 1=diffuse, 2=focal |
|
|
15. Perivascular rarefaction |
|
|
16. Dilation of perivascular spaces |
|
|
17. Perivascular macrophages |
|
|
18. Fibrohyalinotic vascular changes |
|
|
|
RIGHT |
LEFT |
19. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
20. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
21. SP (neuritic): scoring 0-4 |
|
|
22. SP (diffuse): scoring 0-4 |
|
|
23. Lewy Bodies: scoring 0-2 |
|
|
P. CALCARINE REGION |
||
AMYLOID ANGIOPATHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
1. Intraparenchymal |
|
|
2. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
Cortex |
||
3. Cavitated |
|
|
4. Non-cavitated |
|
|
5. Acute |
|
|
6. Microhemorrhages |
|
|
Subcortical White Matter |
||
|
RIGHT |
LEFT |
7. Cavitated |
|
|
8. Non-cavitated |
|
|
9. Acute |
|
|
10. Microhemorrhages |
|
|
11. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
12. Spongiform Change: |
|
|
Subcortical Deep White Matter: scoring 0-3 |
||
|
RIGHT |
LEFT |
13. Myelin Loss
14. If yes: 1=diffuse, 2=focal |
|
|
15. Perivascular rarefaction |
|
|
16. Dilation of perivascular spaces |
|
|
17. Perivascular macrophages |
|
|
18. Fibrohyalinotic vascular changes |
|
|
COUNTS: |
||
|
RIGHT |
LEFT |
19. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
20. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
21. SP (neuritic): scoring 0-4 |
|
|
22. SP (diffuse): scoring 0-4 |
|
|
P2. AREA 18/19 |
||
AMYLOID ANGIOPATHY: scoring 0-4 |
||
|
RIGHT |
LEFT |
1. Intraparenchymal |
|
|
2. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
Cortex |
||
3. Cavitated |
|
|
4. Non-cavitated |
|
|
5. Acute |
|
|
6. Microhemorrhages |
|
|
Subcortical White Matter |
||
|
RIGHT |
LEFT |
7. Cavitated |
|
|
8. Non-cavitated |
|
|
9. Acute |
|
|
10. Microhemorrhages |
|
|
11. Patchy Gliosis (GFAP): scoring 0-3 |
|
|
12. Spongiform Change: |
|
|
Subcortical Deep White Matter: scoring 0-3 |
||
|
RIGHT |
LEFT |
13. Myelin Loss
14. If yes: 1=diffuse, 2=focal |
|
|
15. Perivascular rarefaction |
|
|
16. Dilation of perivascular spaces |
|
|
17. Perivascular macrophages |
|
|
18. Fibrohyalinotic vascular changes |
|
|
AREA 18 COUNTS: |
||
|
RIGHT |
LEFT |
19. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
20. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
21. SP (neuritic): scoring 0-4 |
|
|
22. SP (diffuse): scoring 0-4 |
|
|
AREA 19 COUNTS: |
||
|
RIGHT |
LEFT |
23. NFTs (Biel): scoring 0,1,2,3,4 |
|
|
24. NFTs (AT8): scoring 0,1,2,3,4 |
|
|
25. SP (neuritic): scoring 0-4 |
|
|
26. SP (diffuse): scoring 0-4 |
|
|
Q. Periventricular White Matter Microvascular Change: Scoring 0-4 |
||
Level of CAP-Frontal Horn |
||
|
RIGHT |
LEFT |
1. Gliosis |
|
|
2. Fibrohyalinotic Vascular Change |
|
|
3. Myelin Loss |
|
|
Level of Amygdala-Frontal Horn |
||
RIGHT |
RIGHT |
LEFT |
4. Gliosis |
|
|
5. Fibrohyalinotic Vascular Change |
|
|
6. Myelin Loss |
|
|
Level of Red Nucleus-Frontal Horn |
||
RIGHT |
RIGHT |
LEFT |
7. Gliosis |
|
|
8. Fibrohyalinotic Vascular Change |
|
|
9. Myelin Loss |
|
|
Level of Splenium-Occipital Horn |
||
RIGHT |
RIGHT |
LEFT |
10. Gliosis |
|
|
11. Fibrohyalinotic Vascular Change |
|
|
12. Myelin Loss |
|
|
R. HIPPOCAMPAL FORMATION |
||
|
RIGHT |
LEFT |
1. Acute Ischemic Change |
|
|
2. NFTs @ 200X (AT8): count CA1 |
|
|
3. NFTs @ 200X (Biel): count CA1 |
|
|
4. > 50% Ghosts scoring: 0=no 1=yes |
|
|
5. NFTs @ 200X (AT8): count CA2 |
|
|
6. NFTs @ 200X (Biel): count CA2 |
|
|
7. SPs (Neuritic) @ 100X |
|
|
8. SPs (Diffuse) @ 100X |
|
|
9. Hippocampal sclerosis: scoring 0-4 Scoring: 0=none; 1= CA1 only; 2=CA1/Subiculum; 3=Subiculum fields/Prosubiculum; 4= All CA fields, subiculum/Prosubiculum |
|
|
NEURONAL LOSS AND GLIOSIS: scoring 0-4 |
||
|
RIGHT |
LEFT |
10. CA4 |
|
|
11. CA3 |
|
|
12. CA2 |
|
|
13. CA1 |
|
|
14. Hemosidern-laden macrophages: scoring 0-4 |
|
|
15. Subiculum |
|
|
16. Hippocampal Ferruginization |
|
|
17. Lewy Bodies, CA1, synuclein |
|
|
18. Synuclein positive neuritis in CA2/3 |
|
|
AMYLOID ANGIOPATHY |
||
19. Intraparenchymal |
|
|
20. Leptomeningeal |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
21. Cavitated |
|
|
22. Non-cavitated |
|
|
23. Acute |
|
|
24. Microhemorrhages |
|
|
S. ENTORHINAL CORTEX |
||
|
RIGHT |
LEFT |
1. Neuronal Loss and Gliosis: scoring 0-4 |
|
|
2. Astrocytosis |
|
|
3. Spogiform change |
|
|
4. NFTs @ 200X (AT8): |
|
|
5. NFTs @ 200X (Biel): |
|
|
6. > 50% ghosts scoring: 0=no, 1=yes |
|
|
7. SPs @100X Neuritic |
|
|
8. SPs @ 100X Diffuse |
|
|
9. Lewy Bodies |
|
|
10. Ballooned neurons (aB crystalline) |
|
|
11. Argyrophilic Grains |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
12. Cavitated |
|
|
13. Non-cavitated |
|
|
14. Acute |
|
|
15. Microhemorrhages |
|
|
16. Transentorhinal NFTs: scoring 0-4 |
|
|
17. Lewy Bodies |
|
|
T. AMYGDALA |
||
|
RIGHT |
LEFT |
1. Neuronal Loss and Gliosis: scoring 0-4 |
|
|
2. Astrocytosis |
|
|
3. NFTs @ 200X (AT8): |
|
|
4. NFTs @ 200X (Biel): |
|
|
5. > 50% ghosts scoring: 0=no, 1=yes |
|
|
6. SPs @100X Neuritic |
|
|
7. SPs @ 100X Diffuse |
|
|
8. Lewy Bodies |
|
|
9. Lewy Neurites |
|
|
10. Ballooned neurons (aB crystalline) |
|
|
11. Argyrophilic Grains |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
12. Cavitated |
|
|
13. Non-cavitated |
|
|
14. Acute |
|
|
15. Microhemorrhages |
|
|
U. SUBSTANTIA INNOMINATA (nuc basalis Meynert) |
||
|
RIGHT |
LEFT |
1. Neuronal Loss |
|
|
2. NFTs (AT8) |
|
|
3. Lewy Bodies |
|
|
4. Lewy Neurites |
|
|
CAUDATE/PUTAMEN |
||
|
RIGHT |
LEFT |
5. Astrocytosis |
|
|
6. Patchy Gliosis: scoring 0-3 |
|
|
7. Perivascular rarefaction |
|
|
8. Dilation of perivascular spaces |
|
|
9. Perivascular macrophages |
|
|
10. Arteriolosclerosis |
|
|
11. Perivascular Hemosiderin/Pigment |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
12. Cavitated |
|
|
13. Non-cavitated |
|
|
14. Acute |
|
|
15. Microhemorrhages |
|
|
V. GLOBUS PALLIDUS |
||
|
RIGHT |
LEFT |
1. Patchy Gliosis: scoring 0-3 |
|
|
2. Perivascular rarefaction |
|
|
3. Dilation of perivascular spaces |
|
|
4. Perivascular macrophages |
|
|
5. Arteriolosclerosis |
|
|
6. Pigment deposition |
|
|
7. Ferruginization of Blood Vessels |
|
|
8. NFTs (AT8) |
|
|
MICROINFARCTS: number |
||
|
RIGHT |
LEFT |
9. Cavitated |
|
|
10. Non-cavitated |
|
|
11. Acute |
|
|
12. Microhemorrhages |
|
|
THALAMUS |
|||
|
RIGHT |
LEFT |
|
13. Patchy Gliosis: scoring 0-3 |
|
|
|
14. Perivascular rarefaction |
|
|
|
15. Dilation of perivascular spaces |
|
|
|
16. Perivascular macrophages |
|
|
|
17. Arteriolosclerosis |
|
|
|
18. NFTs (AT8) |
|
|
|
MICROINFARCTS: number |
|||
|
RIGHT |
LEFT |
|
19. Cavitated |
|
|
|
20. Non-cavitated |
|
|
|
21. Acute |
|
|
|
22. Microhemorrhages |
|
|
|
SUBTHALAMIC NUCLEUS |
|||
|
RIGHT |
LEFT |
|
23. Neuronal Loss |
|
|
|
24. NFTs (AT8) |
|
|
|
W. BRAINSTEM |
|
MICROINFARCTS: number |
|
1. Cavitated |
Not entered |
2. Non-cavitated |
|
3. Acute |
|
4. Microhemorrhages |
|
Dorsal and Median Raphe |
|
5. Neuronal Loss: scoring 0-4 |
|
6. NFTs (AT8) |
|
7. Lewy Bodies |
|
8. Pons: Microinfarcts |
|
Locus Coeruleus |
|
9. Neuronal Loss: scoring 0-4 |
|
10. NFTs (AT8) |
|
11. Lewy Bodies |
|
Basis Pontis |
|
12. NFTs (AT8) |
|
13. Microinfarcts |
Not Entered |
14. Wallerian Degeneration: Scoring 0=No, 1=Yes |
|
15. Medulla: Microinfarcts |
|
Dorsal Nucleus of the Vagus: scoring 0-4 |
|
16. Lewy Bodies |
|
17. Lewy Neurites |
|
Inferior Olives: scoring 0-4 |
|
18. Neuronal Loss: scoring 0-4 |
|
19. NFTs (AT8) |
|
Pyramid |
|
20. Wallerian Degeneration: Scoring 0=No, 1=Yes |
|
X. CEREBELLUM |
|
Cortex |
|
1. Spongiform Change |
|
2. SPs: Neuritic |
|
3. SPs: Diffuse |
|
MICROINFARCTS: number |
|
4. Cavitated |
|
5. Non-cavitated |
|
6. Acute |
|
7. Microhemorrhages |
|
Dentate Nucleus: scoring 0-4 |
|
8. Neuronal Loss: scoring 0-4 |
|
9. NFTs (AT8) |
|
Purkinje Cells: scoring 0-4 |
|
10. Neuronal Loss |
|
11. Spheroids |
|
White Matter: scoring 0-4 |
|
12. Myelin Loss:
13. If yes, 1=diffuse, 2=focal |
|
14. Ferruginization |
|
MICROINFARCTS: number |
|
15. Cavitated |
|
16. Non-cavitated |
|
17. Acute |
|
18. Microhemorrhages |
|
Y. SUMMARY SCORES: ITEMS 3 1-24 ARE CALCULATED IN THE DATABASE (will not be on form!!!) |
|||
|
RIGHT |
LEFT |
|
1. Total AT8 NFT |
|
|
|
2. Cortical AT8 NFT |
|
|
|
3. MTL AT8 NFT |
|
|
|
4. Total Biel NFT |
|
|
|
5. Cortical Biel NFT |
|
|
|
6. MTL Biel NFT |
|
|
|
7. Total Diffuse Plaques |
|
|
|
8. Cortical Diffuse Plaques |
|
|
|
9. MTL Diffuse Plaques |
|
|
|
10. Total Neuritic Plaques |
|
|
|
11. Cortical Neuritic Plaques |
|
|
|
12. MTL Neuritic Plaques |
|
|
|
MICROINFARCTS: number |
|||
|
RIGHT |
LEFT |
|
Cortex |
|||
13. Total Cavitated |
|
|
|
14. Total Non-cavitated |
|
|
|
15. Total Acute |
|
|
|
16. Total Microhemorrhages |
|
|
|
Subcortical White Matter |
|||
|
RIGHT |
LEFT |
|
17. Total Cavitated |
|
|
|
18. Total Non-cavitated |
|
|
|
19. Total Acute |
|
|
|
20. Total Microhemorrhages |
|
|
|
21. Deep Nuclei Microinfarcts |
|
|
22. MTL Microinfarcts |
|
|
23. Total Cortical Lewy Body |
|
|
24. Brainstem Lewy Body |
|
|
Z. DIAGNOSIS (PAGES 33-38 ARE SUMMARY ITEMS FOR NACC: COMPLETED BY DR. ANN MCKEE) |
|
7. Does the brain have any gross or microscopic pathology (including any Alzheimer type pathology such as senile plaques and neurofibrillary tangles)?
Scoring: 1=YES; 2=NO |
|
ALZHEIMER’S DISEASE. For all brains in which there is any degree of Alzheimer type pathology (ranging from a few senile plaques and neurofibrillary tangles to advanced Alzheimer’s Disease). Please indicate the nature of the pathology according to commonly used pathologic criteria. |
|
8A. NIA/Reagan Institute neuropathological criteria used: Scoring: 1=High likelihood of dementia being due to Alzheimer’s disease 2=Intermediate likelihood of dementia being due to Alzheimer’s disease 3=Low likelihood of dementia being due to Alzheimer’s disease 4=Criteria not met 5=Not done 9=Missing/unknown |
|
8B. CERAD neuropathological criteria used: Scoring: 1=Definite Alzheimer’s disease 2=Probable Alzheimer’s disease 3=Possible Alzheimer’s disease 4=Criteria not met 5=Not done 9=Missing/unknown |
|
8C. ARDA/Khachaturian neuropathological criteria used: Scoring: 1=Alzheimer’s disease 2=Criteria not met 3=Not done 9=Missing/unknown |
|
8D. Other or unspecified neuropathological criteria used (e.g., Tierney, etc.): Scoring: 1= Alzheimer’s disease, unspecified 2=Criteria not met 3=Not done 9=Missing/Unknown |
|
NEUROFIBRILLARY PATHOLOGY. For all brains in which topographic staging of neurofibrillary degeneration was done, please, indicate the stage with a number from 1-7. If Braak staging was not done, use number 8. |
|
9. Braak & Braak Neurofibrillary Stage: Scoring: 1=Stage I 6=Stage V1 2=Stage II 7=Neurofibrillary degeneration not present 3=Stage III 8=Not assessed 4=Stage IV 9=Missing/unknown 5=Stage |
|
PLAQUE SCORE. For the most severely affected cortical region, please indicate the plaque score. Please use the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) standards for sparse, moderate, and frequent. |
|
10. Neuritic plaques (plaques with argyrophilic dystrophic neuritis with or without dense amyloid cores). Scoring: 1=Frequent neuritic plaques 2=Moderate neuritic plaques 3=Sparse neuritic plaques 4=No neuritic plaques 5=Not assessed 9=Missing/unknown |
|
11. Diffuse plaques (plaques with non-compact amyloid and no apparent dystrophic neurites). Scoring: 1= Frequent diffuse plaques 2=Moderate diffuse plaques 3=Sparse diffuse plaques 4=No diffuse plaques 5=Not assessed 9=Missing/unknown |
|
12. Is ischemic, hemorrhagic or vascular pathology present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12A. Are one or more large artery cerebral infarcts present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12B. Are one or more cortical, microinfarcts (including “granular atrophy”) present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12C. Are one or more lacunes (small artery infarcts and/or hemorrhages) present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12D. Are single or multiple hemorrhages present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12E. Is subcortical arteriosclerotic leukoencephalopathy present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12F. Is cortical laminar necrosis present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12G. Is medial temporal lobe sclerosis (including hippocampal sclerosis) present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12H. Is atherosclerotic vascular pathology (of the circle or Willis) present? Scoring: 1=NONE; 2=MILD; 3=MODERATE; 4=SEVERE; 5=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12I. Is arteriosclerosis (small parenchymal arteriolar disease) present? Scoring: 1=NONE; 2=MILD; 3=MODERATE; 4=SEVERE; 5=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12J. Is amyloid angiopathy present? Scoring: 1=NONE; 2=MILD; 3=MODERATE; 4=SEVERE; 5=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12K. Is another type of angiopathy (e.g., CADASIL or arteritis) present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
12L. Is there other pathology related to ischemic or vascular diseases not previously specified present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
LEWY BODY PATHOLOGY. For all brains in which Lewy bodies are detected, indicate the presence and distribution of Lewy-related pathology. This classification is independent of the clinical presentation, which may be variable and include Parkinsonism, dementia, psychosis, sleep disorders, etc. |
|
13A. Pathology is consistent with criteria of Consortium on Dementia with Lewy Bodies for: Scoring: 1=Lewy body pathology, brainstem predominant type 2=Lewy body pathology, intermediate or transitional (limbic) type 3=Lewy body pathology, diffuse (neocortical) type 4=Lewy body pathology, unspecified or not further assessed 5=No Lewy bodies 6=Not assessed 9=Missing/unknown |
|
13B. Likelihood that DLB Clinical Syndrome due to DLB Pathology: Scoring: 1 = Low 2 = Intermediate 3 = High 6 = N/A 9 = Missing/unknown |
|
FRONTOTEMPORAL DEGENERATIONS (FTD). (Use this for non-Alzheimer degenerative disorders that commonly have the brunt of cortical changes in frontal and temporal lobes, but may involve other cortical and subcortical regions and have variable clinical presentations, including frontal lobe dementia, progressive aphasia, progressive apraxia, etc.) |
|
14A. Pick’s disease: Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14B. Corticobasal degeneration: Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14C. Progressive supranuclear palsy: Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14D. Frontotemporal dementia and Parkinsonism with tau-positive or argyrophilic inclusions: Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14E. Taupathy, other (e.g., tangle-only dementia and argyrophilic grain dementia): Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14F. FTD with ubiquitin-posistive (tau-negative) inclusions: Scoring: 1=FTD with motor neuron disease 2=FTD without motor neuron disease 3=None present 4=Not assessed 9=Missing/unknown |
|
14G. Is there FTD with no distinctive histopathology (tau-negative, ubiquitin-negative, and no argyrophilic inclusions)? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
14H. Was FTD “not otherwise specified” present (e.g., “Immunostaining for ubiquitin and tau not done”)? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
PRION-RELATED DISORDERS |
|
15A. Is Creutzfeldt-Jakob disease or variant CJD present? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
15B. Are other prion diseases present (e.g., Gerstmann-Straussler syndrome)? Scoring: 1=YES; 2=NO; 3=NOT ASSESSED; 9=MISSING/UNKNOWN |
|
OTHER MAJOR PATHOLOGIC DISORDERS (e.g., infectious, immunologic metabolic, neoplastic, toxic or degenerative). |
|
16A. Are other major pathologic disorders present (not addressed by questions 8-15)? Scoring: 1=YES; 2*=NO; 3*=NOT ASSESSED; 9=MISSING/UNKNOWN If 2, 3, or 9, go to #17A |
|
16B. If 16A is yes, then specify below (one disorder per line). 1. __________________________________________________________________ 2. __________________________________________________________________ 3. __________________________________________________________________
|
|
17A. Family history information relevant to neuropathologic diagnosis. Choose one of the following categories that most accurately describes the family information available: Scoring: 1*=Family history of similar neurodegenerative disorder 2=Family history of other (dissimilar) neurodegenerative disorder 3*=No family history of similar or dissimilar neurodegenerative disorder 4=Family history of both similar and dissimilar neurodegenerative disorder 9*=Family history unknown or not available. *If 1, 3, or 9 go to # 18A. |
|
17B. If 17A is 2 or 4 then specify: ____________________________________________________________
|
|
GENETIC VARIANTS OR POLYMORPHISMS. For each of the following three common genetic variants or polymorphisms, choose the patient’s genotype, if known; select “not available or not assessed” if unknown: |
|
18A. Apolipoprotein-E: Scoring: 1=e3, e3 5=e4, e2 2=e3, e4 6=e2, e2 3=e3, e2 9=Missing/unknown/not assessed 4=e4, e4 |
|
18B. Tau haplotype: Scoring: 1=H1, H1 4=Other polymorphism (e.g., AO) 2=H1, H2 9= Missing/unknown/not assessed 3=H2, H2 |
|
18C. PRNP codon 129: Scoring: 1=M, M 2=M, V 3=V, V 9= Missing/unknown/not assessed |
|
19. GENETIC OR CHROMOSOMAL ABNORMALITIES. Choose below the one known genetic or chromosomal abnormality that best describes the subject: Scoring: 1=APP mutation 9=Notch 3 mutation (CADASIL) 2=PS1 mutation 10=Other known genetic mutation (e.g., Abri, neuroserpin) 3=PS2 mutation 11=Down Syndrome 4=Tau mutation 12=Other chromosomal abnormality 5=α-Synuclein mutation 13=No known genetic or chromosomal abnormality 6=Parkin mutation 50=Not assessed 7=PRNP mutation 99=Missing/unknown 8=Huntingtin mutation |
|
20. What are the primary and contributing pathologic diagnoses or features which you judge to be responsible for the subject’s cognitive status? (NOTE: Mark only one diagnosis as “primary”; any number maybe marked as “contributing”) |
||
Primary |
Contributing |
Diagnosis |
A1 |
A2 |
Normal Brain (NC) |
B1 |
B2 |
AD pathology present but insufficient for AD diagnosis |
C1 |
C2 |
Alzheimer disease (AD) |
D1 |
D2 |
Lewy body disease, with or without AD |
E1 |
E2 |
Vascular disease |
F1 |
F2 |
FTLD |
G1 |
G2 |
Hippocampal sclerosis |
H1 |
H2 |
Prion-associated disease |
I1 |
I2 |
Other (specify): |
J1 |
J2 |
Other (specify): |
K1 |
K2 |
Other (specify): |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Pilot Study to Develop a Biorepository Associated with the National ALS Registry |
| Author | MLG1 |
| File Modified | 0000-00-00 |
| File Created | 2021-08-17 |
© 2026 OMB.report | Privacy Policy