CMS Crosswalk 111618
CMS Cross walk 11.16.18.docx
The National Healthcare Safety Network (NHSN)
CMS Crosswalk 111618
OMB: 0920-0666
0920-0666 NHSN
NHSN 0920-0666 PRA PACKAGE REVIEW
Review how NHSN is accounting for CMS reducing reporting requirements
As part of the CMS Meaningful Measures initiative, CMS finalized proposals during the summer and fall of 2018 to discontinue the required reporting of measures in the End-Stage Renal Disease Quality Incentive Program (QIP) and the Long-Term Care Hospital (LTCH), Inpatient Rehabilitation Facility (IRF), Inpatient Psychiatric Facility (IPF), Ambulatory Surgery Center (ASC), and Outpatient quality reporting programs.. While some facilities will continue to report these measures voluntarily or in fulfillment of state mandates, the overall burden for completing the forms associated with the measures has decreased. NHSN intends to make resulting revisions to its data collection tools in this ICR. The attached chart details the recent CMS final regulations that implement the discontinuation of required reporting of select measures in some of the quality reporting programs. For each of those measures, the associated forms are listed with the burden reduction in this ICR. Forms (57.112) (57.127) (57.128) and (57.203) likely, will all have an associated burden decrease based on adjustments made to the number of facilities that are no longer required for reporting as part of CMS quality reporting programs. However, multiple states still require mandated HAI reporting. Therefore, there is significant variability in the voluntary reporting by many NHSN facilities.
Five Step enrollment for NHSN facilities and its relationship to ICR.
The Five-step enrollment webpage is a tool used by NHSN users to provide an understanding of the process for gaining access to NHSN. The time estimates included on this page were developed based on some forms in this ICR as well as additional materials and processes that are exempt from the ICR. NHSN is working with developers to update the estimates posted on the website.
A brief overview of NHSN Changes included in ICR.
NHSN has updated the burden table to reflect direct burden adjustments in highlighted text. Also, the burden table was updated to reflect the direct burden impact for each form and the annual change for this ICR. Finally, Supporting Statement A has been updated to reflect the items that are accompanied by attachments as requested by OMB.
Reporting Program |
Measure |
Effective date |
Final rule |
NHSN FORMS Impacted |
Burden Decrease |
Long-Term Acute Care Hospital (LTCHQR) |
NHSN Facility-wide Inpatient Hospital-onset Methicillin-resistant Staphylococcus aureus (MRSA) Bacteremia Outcome Measure (NQF #1716) (beginning with the FY 2020 LTCH QRP) |
October 1, 2018 (3Q2018 is the last reporting period – deadline of February 15, 2019) |
Fiscal Year (FY) 2019 Medicare Hospital Inpatient Prospective Payment System (IPPS) and Long-Term Acute Care Hospital (LTCH) Prospective Payment System Final Rule (CMS-1694-F) published August 2, 2018 |
57.127
|
2,070 |
57.128 |
27,600 |
||||
NHSN Ventilator-Associated Event (VAE) Outcome Measure (beginning with the FY 2020 LTCH QRP) |
57.112 |
25,872 |
|||
Inpatient Rehabilitation Facility (IRFQR) |
NHSN Facility-wide Inpatient Hospital-onset Methicillin-resistant Staphylococcus aureus (MRSA) Bacteremia Outcome Measure (NQF #1716) (beginning with the FY 2020 IRF QRP) |
October 1, 2018 (3Q2018 is last reporting period – deadline of February 15, 2019) |
Fiscal Year 2019 Medicare Inpatient Rehabilitation Facility Prospective Payment System Final Rule (CMS-1688-F) published July 31, 2018 |
57.127
|
4,350 |
57.128 |
58,000 |
||||
Inpatient Psychiatric Facility (IPFQR) |
Influenza Vaccination Coverage Among Healthcare Personnel (NQF #0431) |
2018/2019 flu season |
FY 2019 Final Medicare Payment and Quality Reporting Updates for Inpatient Psychiatric Facilities (CMS-1690-F) published July 31, 2018 |
57.203** |
1,417 |
Ambulatory Surgical Center (ASCQR) |
Influenza Vaccination Coverage Among Healthcare Personnel (ASC-8) |
2018/2019 flu season |
Medicare Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System changes for 2019 (CMS-1695-FC) published Nov. 2, 2018 |
57.203** |
1,417 |
Outpatient Hospitals (OQR) |
Influenza Vaccination Coverage Among Healthcare Personnel (OP-27) |
57.203** |
1,417 |
||
ESRD Quality Incentive Program (QIP) |
Healthcare Personnel Influenza Vaccination |
2018/2019 flu season |
CMS
Updates to Policies and Payment Rates for the End-Stage Renal
Disease Prospective Payment System, the Durable Medical Equipment,
Prosthetics, Orthotics, and Supplies (DMEPOS) Competitive Bidding
Program; DMEPOS Fee Schedule Amounts, End-Stage Renal Disease
Quality Incentive Program; and Payment for Renal Dialysis Services
Furnished to Individuals with Acute Kidney Injury |
57.203** |
1,417 |
**Note: Form 57.203 removed from this ICR. The form is not subject to PRA approval due to the statutory waiver for immunization-related work.
5 STEP Enrollment Pg.1
T
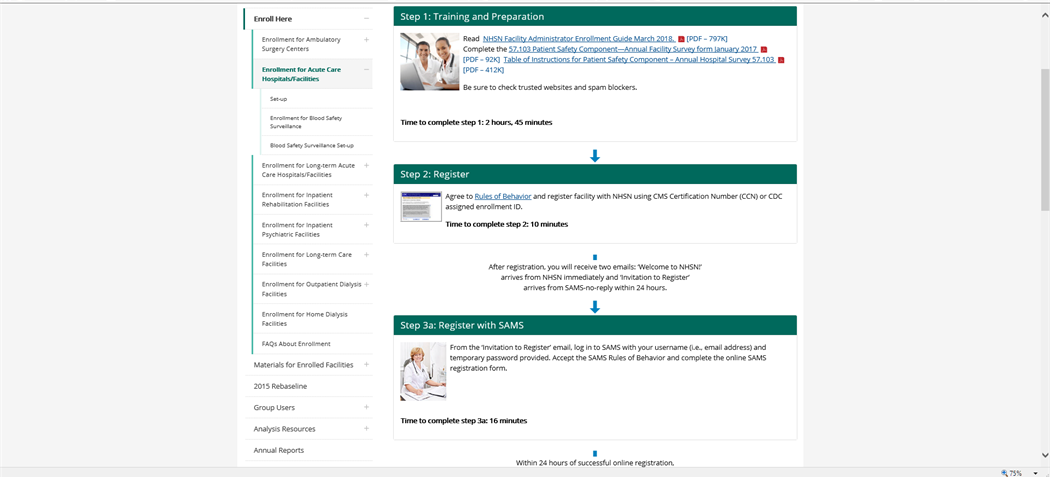 raining
and Preparation
raining
and Preparation
Reading the NHSN Facility Administrator Enrollment Guide is estimated at 35 minutes
57.103 Annual PS Facility Survey – 75 minutes
Table of Instructions – accompanies form 57.103 with instructions to complete
NHSN will update time to complete step #1 from 2 hours and 45 minutes to 1 hour and 50 minutes on the webpage
 Register
Register
Read NHSN Rules of Behavior – 30 minutes
Complete 57.100 (NHSN Registration Form) – 5 minutes
Complete 57.105 (NHSN Group Contact Information) – 5 minutes
NHSN will update time to complete step #2 from 10 minutes to 40 minutes on the webpage
3a. Register with SAMS
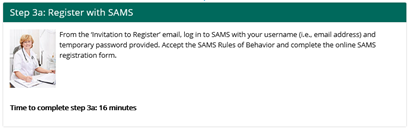
SAMS estimates that it takes 2 minutes to register.
NHSN will update the time to complete step #3a from 16 minutes to 2 minutes on the webpage.
3b. Complete and Submit
Identity Proofing Verification
SAMS estimates that it takes 8 minutes to complete this portion of the registration.
NHSN will update the time to complete step #3b from 35 minutes to 8 minutes on the webpage.
Submit NHSN Forms Electronically

Complete form 57.101 (Facility Contact Information Form) – 10 minutes
NHSN will adjust estimate for step #4 from 32 minutes to 10 minutes on the webpage.
Submit Consent

NHSN Agreement to Participate and Consent form is not required to be OMB approved because it is not a data collection form tool.
NHSN will update time to complete Step #5 from 5 minutes to 10 minutes on the webpage.
NHSN Burden Table
Form Number & Name |
No. of Respondents |
No. of Responses per Respondent |
Avg. Burden per Response (Hours) |
Total Burden (Hours) |
Total burden change from previous Year |
Required by a CMS Reporting program |
The requirement for NHSN participation or state reporting |
Burden change (Hours Increase or Decrease) |
57.100 NHSN Registration Form |
2,000 |
1 |
5/60 |
167 |
0 |
Yes |
This form must be completed during NHSN enrollment, which is required for CMS reporting |
|
57.101 Facility Contact Information |
2,000 |
1 |
10/60 |
333 |
0 |
Yes |
This form must be completed during NHSN enrollment, which is required for CMS reporting |
|
57.103 Patient Safety Component--Annual Hospital Survey |
5,000 |
1 |
1.17 |
7,500 |
2,500 |
Yes; IQR, LTCHQR, PCHQR |
|
Increase
|
57.105 Group Contact Information |
1,000 |
1 |
5/60 |
83 |
0 |
No |
NHSN requires this form to be completed for NHSN group user registration |
|
57.106 Patient Safety Monthly Reporting Plan |
6,000 |
12 |
15/60 |
18,000 |
0 |
Yes; IQR, LTCHQR, PCHQR |
|
|
57.108 Primary Bloodstream Infection (BSI) |
6,000 |
44 |
33/60 |
145,200 |
0 |
Yes; IQR, LTCHQR, PCHQR |
|
|
57.111 Pneumonia (PNEU) |
1,800 |
72 |
30/60 |
64,800 |
0 |
No |
This form must be completed for Pneumonia events reported to NHSN. The city of Pittsburg in Pennsylvania has required reporting on this measure through NHSN by participating facilities in the state. |
|
57.112 Ventilator-Associated Event |
5,615 |
144 |
28/60 |
377,328 |
25,872 |
No |
|
Decrease |
57.113 Pediatric Ventilator-Associated Event (PedVAE) |
100 |
120 |
30/60 |
6,000 |
0 |
No |
This form is not required, it is in the developmental stages and will be active in 2019. |
|
57.114 Urinary Tract Infection (UTI) |
6,000 |
40 |
20/60 |
80,000 |
0 |
Yes; IQR PCHQR IRFQR LTCHQR |
|
|
57.115 Custom Event |
600 |
91 |
35/60 |
31,850 |
0 |
No |
This form is required by NHSN only when a facility customizes data for their event. This data is optional and for facility-level analysis only. |
|
57.116 Denominators for Neonatal Intensive Care Unit (NICU) |
6,000 |
12 |
4 |
288,000 |
0 |
Yes; IQR |
|
|
57.117 Denominators for Specialty Care Area (SCA)/Oncology (ONC) |
2,000 |
9 |
5.03 |
90,600 |
180,480 |
Yes; IQR |
|
Decrease |
57.118 Denominators for Intensive Care Unit (ICU)/Other locations (not NICU or SCA) |
6,000 |
60 |
5.03 |
1,812,000 |
0 |
Yes; IQR |
|
|
57.120 Surgical Site Infection (SSI) |
6,000 |
36 |
35/60 |
126,000 |
0 |
Yes; IQR, PCHQR |
|
|
57.121 Denominator for Procedure |
6,000 |
540 |
10/60 |
540,000 |
0 |
Yes; IQR, PCHQR |
|
|
57.122 HAI Progress Report State Health Department Survey |
55 |
1 |
45/60 |
41 |
41 |
No
|
This is an optional data collection form and is completed by participating healthcare facilities only if a state or local health department is using NHSN data to conduct/manage their HAI surveillance activities. Data captured will aid in the development of the annual HAI progress report. See Attachment D-2 for detailed justification. |
Increase |
57.123 Antimicrobial Use and Resistance (AUR)-Microbiology Data Electronic Upload Specification Tables |
1,000 |
12 |
5/60 |
1,000 |
650 |
Yes; MU3 |
This form is required by NHSN for facilities that report data through electronic health records and as a part of the Meaningful Use Stage 3 incentive. The antimicrobials that are required to be reported for susceptibility testing were reviewed and updated per the most recent Clinical and Laboratory Standards Institute (CLSI) standards. Attachment D-2 for detailed justification. |
Increase
|
57.124 Antimicrobial Use and Resistance (AUR)-Pharmacy Data Electronic Upload Specification Tables |
2,000 |
12 |
5/60 |
2,000 |
1,200 |
Yes; MU3 |
This form is required by NHSN for facilities that report data through electronic health records and as a part of MU3. Two new antimicrobials were recently approved by FDA and will be used by hospitals for treating infections. By capturing the use of these two new drugs, hospitals will be able to better track use and implement stewardship interventions if needed.
|
Increase |
57.125 Central Line Insertion Practices Adherence Monitoring |
100 |
100 |
25/60 |
4,167 |
0 |
No |
|
|
57.126 MDRO or CDI Infection Form |
6,000 |
72 |
30/60 |
216,000 |
0 |
Yes; IQR, PCHQR |
|
|
57.127 MDRO and CDI Prevention Process and Outcome Measures Monthly Monitoring |
4930 |
24 |
15/60 |
29,580 |
6,420 |
Yes; IQR, PCHQR |
The form is not required by NHSN, and is no longer subject to PRA approval due to the statutory waiver for immunization-related work. |
Decrease |
57.128 Laboratory-identified MDRO or CDI Event |
4930 |
240 |
20/60 |
394,400 |
85,600 |
Yes; IQR, PCHQR |
|
Decrease |
57.129 Adult Sepsis |
50 |
250 |
25/60 |
5,208 |
|
No |
This form is not required by NHSN; this module is in a developmental phase and is expected to be active by 2020 |
|
57.137 Long-Term Care Facility Component – Annual Facility Survey |
2,600 |
1 |
2 |
5,200 |
0 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
|
57.138 Laboratory-identified MDRO or CDI Event for LTCF |
2,600 |
12 |
20/60 |
10,400 |
0 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
|
57.139 MDRO and CDI Prevention Process Measures Monthly Monitoring for LTCF |
2,600 |
12 |
20/60 |
10,400 |
5,200 |
No |
This form is required by NHSN for Health Departments to acess the voluntarily reported data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. |
Increase |
57.140 Urinary Tract Infection (UTI) for LTCF |
2,600 |
14 |
35/60 |
18,200 |
0 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
|
57.141 Monthly Reporting Plan for LTCF |
2,600 |
12 |
5/60 |
2,600 |
0 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
|
57.142 Denominators for LTCF Locations |
2,600 |
12 |
4.17 |
130,000 |
5,200 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
Increase |
57.143 Prevention Process Measures Monthly Monitoring for LTCF |
2,600 |
12 |
5/60 |
2,600 |
0 |
No |
This form is required by NHSN for facilities that voluntarily report data into NHSN’s National Nursing Home Quality Collaborative with CMS to track and prevent Clostridioides difficile infections. The state of Nevada has mandated that all Skilled Nursing Facilities report data to NHSN. |
|
57.150 LTAC Annual Survey |
500 |
1 |
1.17 |
583 |
183 |
Yes; LTCHQR |
|
increase |
57.151 Rehab Annual Survey |
1,200 |
1 |
1.17 |
1,400 |
400 |
Yes; IRFQR |
|
Increase |
57.200 Healthcare Personnel Safety Component Annual Facility Survey |
50 |
1 |
8 |
400 |
0 |
No |
This form is required by NHSN and optional for facilities to report various HPS events |
|
57.203 Healthcare Personnel Safety Monthly Reporting Plan |
0 |
1 |
5/60 |
0 |
1,417 |
No;
|
The number of reporting facilities has been dcreased becase required to report to a CMS program such as IQR, IPF, IRF, LTAC, ASC, and Dialysis. |
Decrease |
57.204 Healthcare Worker Demographic Data |
50 |
200 |
20/60 |
3,333 |
0 |
No |
|
|
57.205 Exposure to Blood/Body Fluids |
50 |
50 |
1 |
2,500 |
0 |
No |
|
|
57.206 Healthcare Worker Prophylaxis/Treatment |
50 |
30 |
15/60 |
375 |
0 |
No |
|
|
57.207 Follow-Up Laboratory Testing |
50 |
50 |
15/60 |
625 |
0 |
No |
|
|
57.210 Healthcare Worker Prophylaxis/Treatment-Influenza |
50 |
50 |
10/60 |
417 |
0 |
No |
|
|
57.300 Hemovigilance Module Annual Survey |
500 |
1 |
1.42 |
708 |
292 |
No |
This form is optional but only required by NHSN when a facility is reporting on their Biovigilance Component (BV) events. |
Decrease |
57.301 Hemovigilance Module Monthly Reporting Plan |
500 |
12 |
1/60 |
100 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.303 Hemovigilance Module Monthly Reporting Denominators |
500 |
12 |
1.17 |
7,000 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.305 Hemovigilance Incident |
500 |
10 |
10/60 |
833 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.306 Hemovigilance Module Annual Survey - Non-acute care facility |
200 |
1 |
35/60 |
117 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.307 Hemovigilance Adverse Reaction - Acute Hemolytic Transfusion Reaction |
500 |
4 |
20/60 |
667 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.308 Hemovigilance Adverse Reaction - Allergic Transfusion Reaction |
500 |
4 |
20/60 |
667 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.309 Hemovigilance Adverse Reaction - Delayed Hemolytic Transfusion Reaction |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.310 Hemovigilance Adverse Reaction - Delayed Serologic Transfusion Reaction |
500 |
2 |
20/60 |
333 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.311 Hemovigilance Adverse Reaction - Febrile Non-hemolytic Transfusion Reaction |
500 |
4 |
20/60 |
667 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.312 Hemovigilance Adverse Reaction - Hypotensive Transfusion Reaction |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.313 Hemovigilance Adverse Reaction - Infection |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.314 Hemovigilance Adverse Reaction - Post Transfusion Purpura |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.315 Hemovigilance Adverse Reaction - Transfusion Associated Dyspnea |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.316 Hemovigilance Adverse Reaction - Transfusion Associated Graft vs. Host Disease |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.317 Hemovigilance Adverse Reaction - Transfusion Related Acute Lung Injury |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.318 Hemovigilance Adverse Reaction - Transfusion Associated Circulatory Overload |
500 |
2 |
20/60 |
333 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.319 Hemovigilance Adverse Reaction - Unknown Transfusion Reaction |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.320 Hemovigilance Adverse Reaction - Other Transfusion Reaction |
500 |
1 |
20/60 |
167 |
0 |
No |
This form is required by NHSN and optional for facilities to report Biovigilance Component (BV) events. The state of Massachusetts mandates reporting BV events into NHSN. |
|
57.400 Outpatient Procedure Component—Annual Facility Survey |
5,000 |
1 |
10/60 |
417 |
0 |
No |
This form is required for Ambulatory Surgery Centers (ASC) that have state-based surgical site infection (SSI) surveillance reporting mandates. There are 36 states that have SSI reporting mandates. |
|
57.401 Outpatient Procedure Component - Monthly Reporting Plan |
5,000 |
12 |
20/60 |
15,000 |
0 |
No |
This form is required for Ambulatory Surgery Centers (ASC) that have state-based surgical site infection (SSI) surveillance reporting mandates. There are 36 states that have SSI reporting mandates. |
|
57.402 Outpatient Procedure Component Same Day Outcome Measures |
1,200 |
25 |
40/60 |
20,000 |
0 |
No |
This form is optional for reporting into NHSN |
|
57.403 Outpatient Procedure Component - Monthly Denominators for Same Day Outcome Measures |
1,200 |
12 |
40/60 |
9,600 |
0 |
No |
This form is optional for reporting into NHSN |
|
57.404 Outpatient Procedure Component – SSI Denominator |
5,000 |
540 |
10/60 |
450,000 |
0 |
No |
This form is required for Ambulatory Surgery Centers (ASC) that have state-based surgical site infection (SSI) surveillance reporting mandates. There are 36 states that have SSI reporting mandates. |
|
57.405 Outpatient Procedure Component - Surgical Site (SSI) Event |
5,000 |
36 |
35/60 |
105,000 |
0 |
No |
This form is required for Ambulatory Surgery Centers (ASC) that have state-based surgical site infection (SSI) surveillance reporting mandates. There are 36 states that have SSI reporting mandates. |
|
57.500 Outpatient Dialysis Center Practices Survey |
7,000 |
1 |
2.12 |
14,817 |
467 |
Yes; ESRD QIP |
|
Increase |
57.501 Dialysis Monthly Reporting Plan |
7,000 |
12 |
5/60 |
7,000 |
0 |
Yes; ESRD QIP |
|
These changes will not have an impact on the overall annual burden for this form. |
57.502 Dialysis Event |
7,000 |
60 |
25/60 |
175,000 |
0 |
Yes; ESRD QIP |
|
|
57.503 Denominator for Outpatient Dialysis |
7,000 |
12 |
10/60 |
14,000 |
0 |
Yes; ESRD QIP |
|
|
57.504 Prevention Process Measures Monthly Monitoring for Dialysis |
2,000 |
12 |
1.42 |
17,000 |
13,000 |
No |
|
Decrease |
57.505 Dialysis Patient Influenza Vaccination |
325 |
75 |
10/60 |
4,063 |
0 |
No |
This form is required by NHSN only when a dialysis facility reports flu data into NHSN |
|
57.506 Dialysis Patient Influenza Vaccination Denominator |
325 |
5 |
10/60 |
271 |
0 |
No |
This form is required by NHSN only when a dialysis facility reports flu data into NHSN |
|
57.507 Home Dialysis Center Practices Survey |
350 |
1 |
30/60 |
175 |
0 |
Yes; ESRD QIP |
|
|
|
Total Estimated Annual Burden (Hours) |
5,276,183 |
228,912 |
|
|
|
||
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Bryan, Odion (CDC/OID/NCEZID) (CTR) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-13 |
© 2025 OMB.report | Privacy Policy