App-based Survey Notifications and Messages
Attachment_04d_app-based_notifications_and_messages.docx
Evaluation of the Food and Drug Administration’s Point-of-Sale Campaign
App-based Survey Notifications and Messages
OMB: 0910-0851
ATTACHMENT 4D: MESSAGES, SCREENSHOTS, NOTIFICATIONS, AND INVITATIONS FOR APP-BASED SURVEY
Notifications: Point of Sale Intervention for Tobacco Evaluation (POSITEv)
App Store Description/Information:
Only participants of the POSITEv research study who have been provided with a user ID and password will be able to log into the app. The app was developed by a company that develops apps to research health and behavior. If you choose to download the smartphone application (or app), it will use your phone’s location services to record the time, date, and your location whenever you go into and exit these kinds of stores. The app will also ask you to complete a short questionnaire (5 minutes or less) three times over the next year and a half. The app will not interact with, obtain information from, or transmit information to any of the other apps installed on the phone. Although the app will access location data from the phone, no location data will be logged except data for the mapped convenience stores. The data obtained from the app will not be used for any purpose except for analysis.
Messages
App Installation Pop-up
“POSITEv” Would Like to Send You Notifications. Notifications may include alerts, sounds, and icon badges. These can be configured in Settings.
Don’t allow
Allow
Allow “POSITEv” to access your location? This app requires location access to track your visits to stores that sell tobacco products as a voluntary part of your participation in the POSITEv research study.
Only While Using the App
Always Allow
Don’t Allow
“POSITEv” has been using your location in the background. Do you want to continue allowing this? This app requires location access to track your visits to stores that sell tobacco products.
Settings
Continue
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
App Enrollment Confirmation E-mail
From: fdastudy@rti.org
Date:
To:
Subject: POSITEv: Please verify your email address
Hello,
Thank you for signing up to be a member of the POSITEv community!
To protect your email address we require that you confirm your email address by clicking the link below:
Thank You,
POSITEv study staff
For more information please email: fdastudy@rti.org
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
Incentive Redemption E-mail
From: fdastudy@rti.org
Date:
To:
Subject: How to redeem your POSITEv study incentive
Hello,
Thank you for completing the Point of Sale Intervention for Tobacco Evaluation (POSITEv) questionnaire!
To redeem your incentive, please complete the instructions below:
[FILL: Instructions for redemption of $5 electronic gift card ]
Thank You,
POSITEv study staff
For more information please email: fdastudy@rti.org
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
App Disabled E-mail Message 1
From: fdastudy@rti.org
Date:
To:
Subject: Please reactivate the POSITEv app
Dear [FNAME [LNAME]]:
Thank you for your earlier participation in the POSITEv study! We noticed (your POSITEv app is not active/your location services are not turned on/your POSITEv app no longer has access to your location services) on your smartphone. Please open the app to turn it on. Your continued participation is critical to the success of this important research.
Questions? Need technical support? Call our project assistance line at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have a question about your rights as a study participant, you can call RTI’s Office of Research Protection toll-free at (866) 214-2043.
I hope you choose to help with this important study. Thank you.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
App Disabled E-mail Message 2
From: fdastudy@rti.org
Date:
To:
Subject: Please TAKE ACTION to reactivate the POSITEv app!
Dear [FNAME[LNAME]]:
You are a valued study participant. We noticed (your POSITEv app is not active/your location services are not turned on/your POSITEv app no longer has access to your location services) on your smartphone. Please open the app to turn it on. If we can assist you with this, please contact our helpdesk for assistance in re-installing the app. Our project assistance line is 1-800-957-6457 and is available between 9 am and 5 pm, Eastern Time, Monday through Friday. We are also available by email at fdastudy@rti.org.
I hope you choose to help with this important study. Thank you.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
App Disabled Text Message 1
Your POSITEv app is not working. Please open the app to turn it on. For help, call 1-800-957-6457 or email fdastudy@rti.org. Text STOP to opt out.
App Disabled Text Message 2
Your POSITEv app is not working. You are a valued study participant. Please open the app to turn it on. For help, call 1-800-957-6457 or email fdastudy@rti.org. Text STOP to opt out.
Delete App E-mail [End of Study]
From: fdastudy@rti.org
Date:
To:
Subject: Please DELETE the POSITEv app
Dear [FNAME[LNAME]]:
Thank you very much for participating in the POSITEv study. Now that the study has ended, we would like you to delete the app so that it no longer collects information from you. Please follow the instructions below to delete the app.
Here are the instructions:
If you have an iPhone:
Tap and hold the POSITEv icon until all the icons start wiggling.
An X will appear at the top left corner of the icon.
Tap the X, then tap Delete.
If you have an Android phone:
Tap and hold the POSITEv icon until the word Uninstall appears at the top of the phone.
Drag the POSITEv icon to the word Uninstall.
Tap OK.
If this method doesn’t work:
Locate and tap the Settings icon.
Select the Apps submenu.
Select the App manager or App info submenu.
Tap the POSITEv app.
Tap Uninstall or Delete.
Questions? Need technical support? Call our project assistance line at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have a question about your rights as a study participant, you can call RTI’s Office of Research Protection toll-free at (866) 214-2043.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
Example Screenshots
Below are some example screenshots that contain elements that will be present in the app. Since the app will be created by the vendor for the purpose of the Point of Sale Intervention for Tobacco Evaluation study (POSITEv), and there will be differences in the display between iPhones and Android phones, these images are intended to provide a general feel (approximation) for the app’s appearance, but are not meant to represent the exact appearance of the app.
Welcome screenshot

Consent screenshot
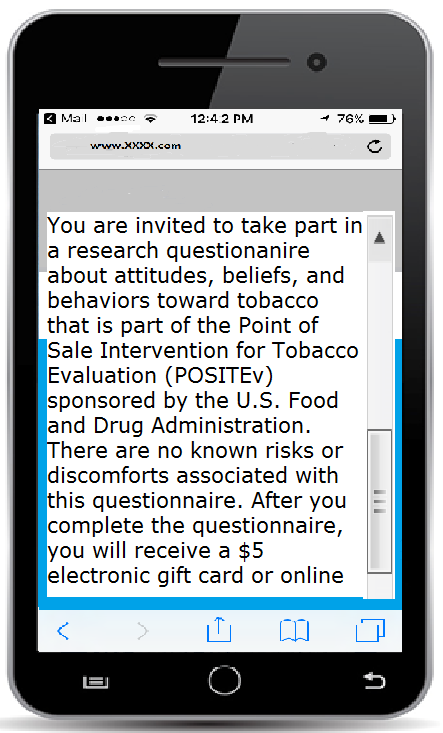
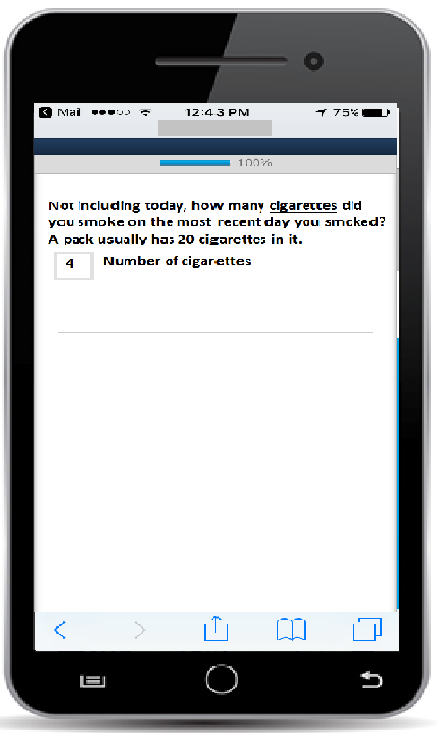
App-based questionnaire item (any number 0-99 can be entered into the box by clicking on it)
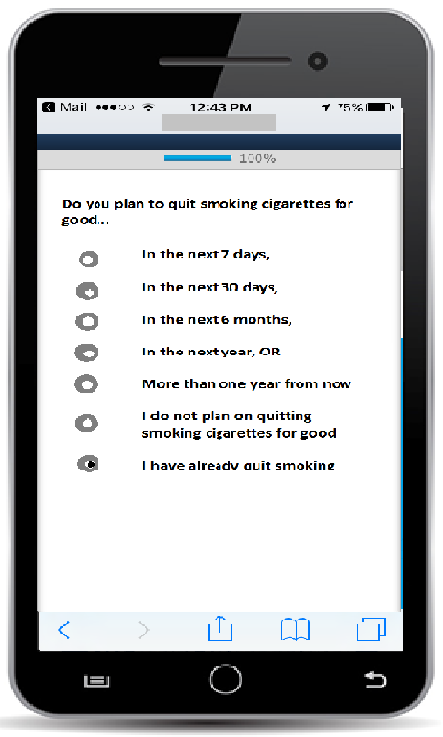
App-based questionnaire item with radio buttons (allows participant to select one response)
End Questionnaire Screenshot

Notifications and Invitations
Push Notifications
Push Notification 1
Complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
[click on] Learn more
Push Notification 2
Time is running out to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
[click on] Learn more
Push Notification 3
Last chance to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
[click on] Learn more
Location Services Notification
Push Notification
POSITEv is not tracking
Turn on location
In-App Notification
App functionality is limited because location services are off.
[click on] Allow location access.
Text Invitations
Text Invitation 1
Please complete the 5 minute POSITEv questionnaire for a $5 electronic gift card! Text STOP to opt out. [questionnaire link here]
Text Invitation 2
Time is running out to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card! Text STOP to opt out. [questionnaire link here]
Text Invitation 3
Last chance to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card! Text STOP to opt out. [questionnaire link here]
Email Invitations
Email Invitation 1
Subject Line: Complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
Dear [FNAME[LNAME]]:
We are contacting you to ask you to participate an app-based questionnaire for the U.S. Food and Drug Administration’s (FDA’s) Center for Tobacco Products’ survey for the Point of Sale Intervention for Tobacco Evaluation (POSITEv). When we interviewed you previously, you agreed to receive information about these short questionnaires.
This is your opportunity to update us on your use of tobacco and your recent tobacco purchases. It will only take about 5 minutes to complete.
You can complete the questionnaire by logging into the following app on your smart phone POSITEv. After completing the questionnaire, you will receive a $5 electronic gift card that can be redeemed from an online vendor.
Your participation is voluntary, and all information will be kept private to the full extent allowable by law.
Questions? Need technical support? Call our project assistance line at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday, or email us at fdastudy@rti.org. If you have a question about your rights as a study participant, you can call RTI’s Office of Research Protection toll-free at (866) 214-2043.
Your help is very important to the success of this study, and I thank you in advance for your consideration.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
Email Invitation 2
Subject Line: Time is running out to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
Dear [FNAME[LNAME]]:
There is still time to complete the app-based questionnaire for the U.S. Food and Drug Administration’s (FDA’s) Center for Tobacco Products’ Point of Sale Intervention for Tobacco Evaluation (POSITEv).
All the information you provide will be kept private, and you can choose to skip any questions you do not want to answer. But, the study will not succeed without your participation.
You can complete the questionnaire by logging into the following app on your smart phone: POSITEv. After completing the questionnaire, you will receive a $5 electronic gift card that can be redeemed from an online vendor.
Questions? Need technical support? Call our project assistance line at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have a question about your rights as a study participant, you can call RTI’s Office of Research Protection toll-free at (866) 214-2043.
I look forward to hearing from you.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
Email Invitation 3
Subject Line: Last chance to complete the 5 minute POSITEv questionnaire for a $5 electronic gift card!
Dear [FNAME[LNAME]]:
You have until [date] to complete the app-based questionnaire for the U.S. Food and Drug Administration’s (FDA’s) Center for Tobacco Products’ Point of Sale Intervention for Tobacco Evaluation (POSITEv).
Your continued participation is critical to the success of this important research.
You can complete the questionnaire by logging into the following app on your smart phone: POSITEv. After completing the questionnaire, you will receive a $5 electronic gift card that can be redeemed from an online vendor.
The information collected will improve our understanding of tobacco use and purchasing behaviors. Your participation is voluntary. All the information you provide will be kept private, and you can choose to skip any questions you do not want to answer.
Questions? Need technical support? Call our project assistance line at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have a question about your rights as a study participant, you can call RTI’s Office of Research Protection toll-free at (866) 214-2043.
I hope you choose to help with this important study. Thank you.
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
New Year’s Message [to be sent to Wave 2 participants who complete the web survey prior to the winter holidays]
Subject Line: Happy New Year from the POSITEv Study Team
Dear [FNAME LNAME]:
Happy New Year! We appreciate your recent participation in the POSITEv study, and hope that you’ll continue to be part of this important study in the coming year.
If you received or purchased a new smartphone over the holiday season, we hope you’ll take a moment to download and log into the POSITEv app again. Your continued participation in the smartphone portion of the POSITEv study helps the FDA to collect information about advertisements adults have seen and their attitudes towards smoking and programs that help smokers who want to quit.
If you are still using the same phone that you used to download the POSITEv app, then no further action is needed today. However, if have a new phone that you’d like to download the app onto, instructions are below.
Here are the instructions for iPhones:
Tap on the “App Store” icon.
Search for POSITEv.
Click the cloud icon to the right of the app name (“POSITEv”). A square should appear with a circle around it in place of the cloud. The circle should slowly change from white to blue.
If you are prompted for your Apple password, enter it.
Once the download is complete, the word “open” will appear to the right of the app name. Click “Open” (or find the app on your home screen).
Click “Allow” when you see the following message: “POSITEv” Would Like to Send You Notifications. Notifications may include alerts, sounds, and icon badges. These can be configured in Settings.”
The first screen you will see is a “Welcome” screen. Swipe from right to left to get to the “Introduction” screen. In the bottom right hand corner, click on the box that reads “Get Started”.
Enter your “RTI ID” and “Password”:
User ID: ___________
Password: fda$tudy
Click “return” on your phone’s keyboard or the button labeled “Sign In” under the login boxes.
After entering your RTI ID and Password the following question should appear via a pop-up: “Allow “POSITEv” to access your location? This app requires location access to track your visits to stores that sell tobacco products as a voluntary part of your participation in the POSITEv research study.”
Please select “Always Allow”
Here are the instructions for Android:
Tap on the “Play Store” icon and select “APPS.”
Search for POSITEv.
If under the search bar you see “showing results for positive” click “search instead for positev”
When you find the app, tap on it and click “Install.”
Click “Open” once download is complete (or find the app on your home screen).
You will see a “Welcome” screen, followed by an “Introduction” screen. On the “Introduction” screen, click on “Get Started”
Enter your “RTI ID” and “Password”:
User ID: ___________
Password: fda$tudy
Please select “Allow”
Thank you again for your participation!
Sincerely,
Matthew Farrelly, PhD
RTI International
To no longer receive our emails, click to unsubscribe
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Dutra, Lauren |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy