Download:
docx |
pdf
MEDICATION
GUIDE
ANDROGEL®
(AN DROW JEL) CIII
(testosterone
gel) 1% for topical use
|
What
is the most important information I should know about ANDROGEL 1%?
ANDROGEL
1% can transfer from your body to others including, children and
women.
Children and women should avoid contact with the unwashed or not
covered (unclothed) areas where ANDROGEL® 1% has been applied
to your skin. Early signs and symptoms of puberty have occurred
in young children who have come in direct contact with
testosterone by touching areas where men have used ANDROGEL 1%.
Signs
and symptoms of early puberty in a child when they come in direct
contact with ANDROGEL 1% may include:
Abnormal
sexual changes:
enlarged
penis or clitoris.
early
growth of hair near the vagina or around the penis (pubic hair).
erections
or acting out sexual urges (sex drive).
Behavior
problems:
Signs
and symptoms in women when they come in direct contact with
ANDROGEL 1% may include:
Stop
using ANDROGEL 1% and call your healthcare provider right away if
you see any signs and symptoms in
a child or a woman that may have happened through accidental
touching of the area where you have applied ANDROGEL 1%.
To
lower the risk of transfer of ANDROGEL 1% from your body to
others, follow these important instructions:
Apply
ANDROGEL 1% only to areas of your shoulders, upper arms, or
stomach area (abdomen) that will be covered by a short sleeve
T-shirt.
Wash
your hands right away with soap and water after applying ANDROGEL
1%.
After
the gel has dried, cover the application area with clothing. Keep
the area covered until you have washed the gel off the
application area well or have showered.
If
you expect to have skin-to-skin contact with another person,
first wash the application area well with soap and water.
If
a child or woman touches the area where you have applied ANDROGEL
1%, that area on the child or woman should be washed well with
soap and water right away.
|
What
is ANDROGEL 1%?
ANDROGEL
1% is a prescription medicine that contains testosterone. ANDROGEL
1% is used to treat adult males who have low or no testosterone
due to certain medical conditions.
ANDROGEL
1% is a controlled substance (CIII) because it contains
testosterone that can be a target for people who abuse
prescription medicines. Keep your ANDROGEL 1% in a safe place to
protect it. Never give your ANDROGEL 1% to anyone else, even if
they have the same symptoms you have. Selling or giving away this
medicine may harm others and is against the law.
ANDROGEL
1% is not meant for use in women.
|
Do
not use ANDROGEL 1% if you:
have
breast cancer.
have
or might have prostate cancer.
are
pregnant or may become pregnant or breast-feeding. ANDROGEL 1%
may harm your unborn or breast-feeding baby.
women
who are pregnant or who may become pregnant should avoid contact
with the area of skin where ANDROGEL 1% has been applied.
|
Before
using ANDROGEL 1%, tell your healthcare provider about all of your
medical conditions, including if you:
have
breast cancer.
have
or might have prostate cancer.
have
urinary problems due to an enlarged prostate.
have
heart problems.
have
liver or kidney problems.
have
problems breathing while you sleep (sleep apnea).
Tell
your healthcare provider about all the medicines you take,
including prescription and over-the-counter medicines, vitamins,
and herbal supplements. Using ANDROGEL 1% with certain other
medicines can affect each other.
Especially,
tell your healthcare provider if you take:
|
How
should I use ANDROGEL 1%?
See
the detailed Instructions
for Use for
information about how to use ANDROGEL 1% at the end of this
Medication Guide.
It
is important that you apply ANDROGEL 1% exactly as your
healthcare provider tells you to.
Your
healthcare provider may change your ANDROGEL 1% dose. Do
not
change your ANDROGEL 1% dose without talking to your healthcare
provider.
Apply
ANDROGEL 1% at the same time each morning. ANDROGEL 1% should be
applied after showering or bathing.
|
What
are the possible side effects of ANDROGEL 1%?
ANDROGEL
1% can cause serious side effects including:
increased
urination at night.
trouble
starting your urine stream.
having
to pass urine many times during the day.
having
an urge to go to the bathroom right away.
having
a urine accident.
being
unable to pass urine or weak urine flow.
Possible
increased risk of prostate cancer.
Your healthcare provider should check you for prostate cancer or
any other prostate problems before you start and while you use
ANDROGEL 1%.
Blood
clots in the legs or lungs.
Signs and symptoms of a blood clot in your leg can include leg
pain, swelling or redness. Signs and symptoms of a blood clot in
your lungs can include difficulty breathing or chest pain.
Possible
increased risk of heart attack or stroke.
In
large doses ANDROGEL 1% may lower your sperm count.
Swelling
of your ankles, feet, or body, with or without heart failure.
This may cause serious problems for people who have heart, kidney
or liver disease.
Enlarged
or painful breasts.
Have
problems breathing while you sleep (sleep apnea).
Call
your healthcare provider right away if you have any of the serious
side effects listed above.
The
most common side effects of ANDROGEL 1% include:
Other
side effects include
more erections than are normal for you or erections that last a
long time.
Tell
your healthcare provider if you have any side effect that bothers
you or that does not go away.
These
are not all the possible side effects of ANDROGEL 1%. For more
information, ask your healthcare provider or pharmacist.
Call
your doctor for medical advice about side effects. You may report
side effects to FDA at 1-800-FDA-1088.
|
General
information about the safe and effective use of ANDROGEL 1%
Medicines
are sometimes prescribed for purposes other than those listed in a
Medication Guide. Do not use ANDROGEL 1% for a condition for which
it was not prescribed. Do not give ANDROGEL 1% to other people,
even if they have the same symptoms you have. It may harm them.
You
can ask your pharmacist or healthcare provider for information
about ANDROGEL 1% that is written for health professionals.
|
What
are the ingredients in ANDROGEL 1%?
Active
ingredient:
testosterone
Inactive
ingredients:
carbomer 980, ethyl alcohol 67.0%, isopropyl myristate, purified
water and sodium hydroxide.
Marketed
by: AbbVie Inc. North Chicago, IL 60064, USA
©
2016 AbbVie Inc.
For
more information, go to www.ANDROGEL.com or call 1-800-633-9110.
|
This
Medication Guide has been approved by the U.S. Food and Drug
Administration. Revised:
DATE
INSTRUCTIONS
FOR USE
ANDROGEL®
(AN DROW JEL) CIII
(testosterone
gel) 1%
for
topical use
Read
this Instructions for Use for ANDROGEL1% before you start using it
and each time you get a refill. There may be new information. This
leaflet does not take the place of talking to your healthcare
provider about your medical condition or treatment.
Applying
ANDROGEL 1%:
Before
applying ANDROGEL 1%, make sure that your shoulders, upper arms or
stomach are clean, dry, and there is no broken skin.
The
application sites for ANDROGEL 1% are the shoulders, upper arms or
stomach area (abdomen) that will be covered by a short sleeve
T-shirt (see Figure A). Do
not apply
ANDROGEL 1% to any other parts of your body such as your penis,
scrotum, chest, armpits (axillae), knees, or back.
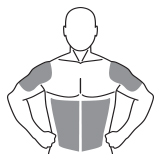
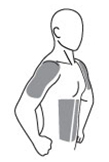
(Figure
A)
Tear
open the packet completely at the dotted line. Squeeze from the
bottom of the packet to the top.
Squeeze
all of the ANDROGEL 1% out of the packet into the palm of your hand.
Apply
ANDROGEL 1% to the application site. You may also apply ANDROGEL 1%
from the packet directly to the application site.
Let
the application areas dry completely before putting on a T-shirt.
ANDROGEL
1% is flammable until dry. Let ANDROGEL 1% dry before smoking or
going near an open flame.
Wash
your hands with soap and water right away after
applying ANDROGEL 1%.
Avoid
showering, swimming, or bathing for at least 5 hours after you apply
ANDROGEL 1%.
How
should I store ANDROGEL 1%?
Store
ANDROGEL 1% at room temperature between 68ºF to 77ºF (20ºC
to 25ºC).
Safely
throw away used ANDROGEL 1% in household trash. Be careful to
prevent accidental exposure of children, women or pets.
Keep
ANDROGEL 1% away from fire.
Keep
ANDROGEL 1% and all medicines out of the reach of children.
This
Instructions for Use has been approved by the U.S. Food and Drug
Administration. Revised: MM/YYYY
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | PATIENT INFORMATION OR MEDICATION GUIDE |
| Author | DMPP |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |

