Consultation with FDA and Termination of Notification - Dispensers
Guidance for Industry on Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification
FINAL GUIDANCE Suspect product notification
Consultation with FDA and Termination of Notification - Dispensers
OMB: 0910-0806

Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification
Guidance for Industry
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Office of Regulatory Affairs (ORA)
XXXX 2015
 Procedural
Procedural
Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification
Guidance for Industry
Additional copies are available from:
Office of Communications
Division of Drug Information, WO51, Room 2201
Center for Drug Evaluation and Research
Food and Drug Administration
10903 New Hampshire Ave., Silver Spring, MD 20993
Phone: 301-796-3400; Fax: 301-847-8714
druginfo@fda.hhs.gov
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
and/or
Office of Communication, Outreach and Development
Center for Biologics Evaluation and Research
Food and Drug Administration
10903 New Hampshire Ave., WO 71, Room 3128
Silver Spring, MD 20993
Phone: 800-835-4709 or 240-402-7800
http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/default.htm
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Office of Regulatory Affairs (ORA)
XXXX 2015
Procedural
TABLE OF CONTENTS
I. INTRODUCTION 1
II. BACKGROUND 2
A. Drug Supply Chain Security Act 2
B. Scope of This Guidance 3
III. Identification of suspect product and, for manufacturers, product with a high risk of illegitimacy 3
A. Specific Scenarios That Could Significantly Increase the Risk of a Suspect Product Entering the Pharmaceutical Distribution Chain 4
1. Trading Partners and Product Sourcing 4
2. Supply, Demand, History, and Value of the Product 5
3. Appearance of the Product 6
B. Recommendations on How Trading Partners Might Identify Suspect Product and Determine Whether the Product Is a Suspect Product as Soon as Practicable 6
C. For Manufacturers: High Risk of Illegitimacy Notifications 8
1. High Risk of Illegitimacy Notification for Products that the Manufacturer Has Reason to Believe Are in an Immediate Trading Partner’s Possession 9
2. Specific High Risks That Could Increase the Likelihood of an Illegitimate Product Entering the U.S. Pharmaceutical Distribution Supply Chain 10
3. Other High Risks as Determined by FDA: High Risk of Illegitimacy Notification Where a Manufacturer has Reason to Believe the Product has Entered the Pharmaceutical Distribution Supply Chain 10
IV. Notification OF ILLEGITIMATE PRODUCT AND PRODUCTS WITH A HIGH RISK OF ILLEGITIMACY 11
A. Notification to FDA 11
1. The following process should be used to notify FDA of illegitimate products: 12
2. The following process should be used by manufacturers to notify FDA of a product with a high risk of illegitimacy: 12
B. Termination of Notification in Consultation With FDA 13
ATTACHMENT A: FORM FDA 3911 16
Guidance for Industry1
Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification
This guidance represents the Food and Drug Administration’s (FDA’s) current thinking on this topic. With the exception of section IV.B, 2 it does not create or confer any rights for or on any person and does not operate to bind the FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
I. INTRODUCTION
This guidance is intended to aid trading partners3 (manufacturers, repackagers, wholesale distributors, and dispensers) in identifying a suspect product and terminating notifications. As of January 1, 2015, a trading partner that determines a product in its possession or control is an illegitimate product must notify the Food and Drug Administration (FDA or Agency) and certain immediate trading partners under section 582 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 360eee), as added by the Drug Supply Chain Security Act (DSCSA). Manufacturers are additionally required under section 582 to notify FDA and certain immediate trading partners after the manufacturer determines or is notified by FDA or a trading partner that there is a high risk that a product is illegitimate. This guidance identifies specific scenarios that could significantly increase the risk of a suspect product entering the pharmaceutical distribution supply chain; provides recommendations on how trading partners can identify a product and determine whether a product is a suspect product as soon as practicable; and sets forth the process by which trading partners should notify FDA of illegitimate product or products with a high risk of illegitimacy, and how they must terminate the notifications, in consultation with FDA.
This guidance does not address all provisions of the DSCSA related to suspect and illegitimate products. As FDA works to implement other provisions of the DSCSA, the Agency intends to issue additional information to support efforts to develop standards, issue guidance and regulations, establish pilot programs, and conduct public meetings.
FDA’s guidance documents, in general, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required. Insofar as section IV.B of this guidance sets forth the process by which trading partners must terminate notifications of illegitimate product and products with a high risk of illegitimacy in consultation with FDA, it has binding effect.4
II. BACKGROUND
On November 27, 2013, the DSCSA (Title II of Public Law 113-54) was signed into law. Section 203 of the DSCSA added new section 582(h)(2) to the FD&C Act, which requires FDA to issue guidance to aid trading partners in identifying a suspect product and terminating notifications. Suspect product is defined in section 581(21) of the FD&C Act as a product for which there is reason to believe it (A) is potentially counterfeit, diverted, or stolen; (B) is potentially intentionally adulterated such that the product would result in serious adverse health consequences or death to humans; (C) is potentially the subject of a fraudulent transaction; or (D) appears otherwise unfit for distribution such that the product would result in serious adverse health consequences or death to humans. Section 582 of the FD&C Act requires trading partners, upon determining that a product in their possession or control is a suspect product, to quarantine the product while they promptly conduct an investigation to determine whether the product is an illegitimate product. Illegitimate product is defined in section 581(8) of the FD&C Act as a product for which credible evidence shows that it is (A) counterfeit, diverted, or stolen; (B) intentionally adulterated such that the product would result in serious adverse health consequences or death to humans; (C) is the subject of a fraudulent transaction; or (D) appears otherwise unfit for distribution such that the product would be reasonably likely to result in serious adverse health consequences or death to humans.5
Section 582 of the FD&C Act requires trading partners, upon determining that a product in their possession or control is illegitimate, to notify FDA and all immediate trading partners (that they have reason to believe may have received the illegitimate product) not later than 24 hours after making the determination. Manufacturers are additionally required under section 582(b)(4)(B)(ii)(II) to notify FDA and immediate trading partners (that the manufacturer has reason to believe may possess a product manufactured by or purported to be manufactured by the manufacturer) not later than 24 hours after the manufacturer determines or is notified by FDA or a trading partner that there is a high risk that the product is illegitimate.
The DSCSA outlines critical steps to build an electronic, interoperable system over the next 10 years that will identify and trace certain prescription drugs as they are distributed within the United States (U.S.). For many years, FDA has been engaged in efforts to improve the security of the drug supply chain to protect U.S. patients from unsafe, ineffective, and poor quality drugs. Since the formation of the first FDA Counterfeit Drug Task Force in 2003, FDA has strongly advocated for a multilayered approach to securing the supply chain. A key component of that approach has been to encourage heightened vigilance and awareness among supply chain partners. The electronic, interoperable system that will be established under the DSCSA will enhance FDA’s ability to help protect U.S. consumers by improving detection and removal of potentially dangerous drugs from the drug supply chain.
Pursuant to section 582(h)(2) of the FD&C Act, this guidance identifies specific scenarios that could significantly increase the risk of a suspect product entering the pharmaceutical distribution chain; provides recommendations on how trading partners can identify a product and determine whether a product is a suspect product as soon as practicable; describes when manufacturers should notify FDA of a high risk that a product is illegitimate; and sets forth the process by which trading partners must terminate notifications in consultation with FDA regarding illegitimate product under section 582(b)(4)(B)(iv), (c)(4)(B)(iv), (d)(4)(B)(iv), and (e)(4)(B)(iv) of the FD&C Act and the process for terminating notifications in consultation with FDA regarding products with a high risk of illegitimacy under section 582(b)(4)(B)(iv). This guidance also addresses how trading partners should notify FDA when they determine that a product in their possession or control is an illegitimate product under section 582(b)(4)(B)(ii)(I), (c)(4)(B)(ii), (d)(4)(B)(ii), and (e)(4)(B)(ii) of the FD&C Act, and how manufacturers should notify FDA regarding products with a high risk of illegitimacy under section 582(b)(4)(B)(ii)(II).
III. Identification of suspect product and, for manufacturers, product with a high risk of illegitimacy
As background, under section 582 of the FD&C Act, trading partners must have systems in place that enable them, upon determining that a product in their possession or control is suspect or upon receiving a request for verification from the FDA that has made a determination that a product within the possession or control of the trading partner is a suspect product, to quarantine suspect product and promptly conduct an investigation, in coordination with other trading partners, as applicable, to determine whether a suspect product is illegitimate.
As trading partners conduct business on a daily basis, they should exercise vigilance, maintain awareness about suspicious activity or potential threats to their supply chain, and devote attention and effort to detect suspect product.
The next two sections of this guidance (A.) identify some specific scenarios that could significantly increase the risk of suspect products entering the pharmaceutical distribution supply chain and (B.) make recommendations to assist trading partners in identifying suspect product and making determinations about whether a product is suspect as soon as practicable. The scenarios contained in this guidance are based on Agency experience with suspect product in the drug supply chain. These examples are illustrative and should be viewed as guidance rather than as an exhaustive list of all potential scenarios that increase the likelihood that a suspect product could enter the pharmaceutical distribution supply chain. Trading partners should consider the surrounding circumstances of any particular scenario they may encounter in determining whether or not a product is suspect, including whether multiple scenarios are present in any given transaction.
Specific Scenarios That Could Significantly Increase the Risk of a Suspect Product Entering the Pharmaceutical Distribution Chain
There may be situations involving trading partners where heightened vigilance would be appropriate. In addition, there could be identifiable characteristics of products that might increase the likelihood that they are suspect products. The following are examples of some specific scenarios that could significantly increase the risk of a suspect product entering the drug supply chain. Thus, trading partners should be particularly diligent when engaging in transactions that involve:
Purchasing from a source new to the trading partner.
Receiving an unsolicited sales offer from an unknown source. Trading partners might receive unsolicited offers or advertisements through an email, a fax, a telephone call, or an in-person sales call from a person or entity with whom they do not have an established business relationship.
Purchasing on the Internet from an unknown source. Trading partners might be searching for a better price on the Internet or for a product that they cannot obtain from their usual source, and might be tempted to turn to a person or entity with whom they do not have an established business relationship.
Purchasing from a source that a trading partner knows or has reason to believe has engaged in questionable or suspicious business practices that could increase the risk of suspect product entering the supply chain, such as:
A trading partner that has been involved in business transactions where they sold or delivered illegitimate product.
A trading partner that has a history of problematic or potentially false transaction histories or pedigrees, such as those that contain misspelled words or incomplete information.
A trading partner that is reluctant to provide a transaction history associated with the product being purchased, or does not do so in a timely manner.
A trading partner that provides transaction information, a transaction statement, and/or transaction history that appears to be incomplete or suspicious.
Product that is generally in high demand in the U.S. market.
Product that is in higher demand because of its potential or perceived relationship to a public health or other emergency (e.g., antiviral drugs).
Product that has a high sales volume or price in the United States.
Product offered at a price that is “too good to be true.”
Product that has been previously or is currently being counterfeited or diverted (e.g., HIV, antipsychotic, or cancer drugs).
Product that has been previously or is currently the subject of a drug shortage (see a list of current drugs in shortage at http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shortages/default.htm and http://www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm for more information).
Product that has been or is the subject of an illegitimate product notification under the DSCSA or other alert or announcement related to drug quality.
Product that has been or is the subject of an FDA counterfeit or cargo theft alert
(See http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/counterfeitmedicine/default.htm and http://www.fda.gov/iceci/criminalinvestigations/ucm182888.htm for more information).
Appearance of a package or a container used for transport (e.g., case or tote) that seems suspicious (e.g., it has a label that contains misspellings or appears different from the standard label for that product in color, font, images, or otherwise).
Package that exhibits unusual or excessive adhesive residues.
Package that contains foreign identification features (such as a different drug identification number where a National Drug Code (NDC) number would be expected).
Package that is missing information, such as the lot number or other lot identification, or the expiration date.
Package that is missing security or anti-counterfeiting technologies normally featured on the FDA-approved product that are easily visible to the eye, such as holograms, color shifting inks, neckbands or watermarks.
Finished dosage form that seems suspicious (e.g., it has a different shape or color from the FDA-approved product, a different or unusual imprint, an unusual odor, or there are signs of poor quality like chips or cracks in tablet coatings or smeared or unclear ink imprints).
Recommendations on How Trading Partners Might Identify Suspect Product and Determine Whether the Product Is a Suspect Product as Soon as Practicable
The following are recommendations for trading partners on ways that they can expeditiously identify suspect product and determine whether the product is suspect (and, after investigation, whether it is illegitimate).. In general, trading partners should exercise due diligence when conducting business and should confirm that all trading partners are authorized. Trading partners should discuss with each other any observations, questions, or concerns they have related to the status of a drug as a suspect product to aid them in determining whether the drug should be considered a suspect product. Trading partners should also contact regulatory authorities, law enforcement, the drug’s manufacturer or other available resources to aid in that determination when additional expertise is called for to make an accurate assessment of the status of a drug as a suspect product. If a trading partner receives a product in a secured transport container or sealed homogenous case, trading partners should examine the appearance of that container as recommended below. If trading partners observe anything suspicious, they should take steps to ascertain whether the product inside the transport container is suspect. Strategies to identify suspect product include, but are not limited to, the following recommendations:
Be alert for offers of product for sale at a very low price or one that is “too good to be true.”
Closely examine the package and the transport container (such as the case or tote):
To look for signs that it has been compromised (e.g., opened, broken seal, damaged, repaired, or otherwise altered). If a trading partner receives a product in a secured transport container or sealed homogenous case, trading partners should examine the appearance of that container to see if anything about that appearance seems suspicious, such as a shrink wrap that has unexpected markings, or a seal that is broken, torn, or repaired.
To see if the package or the transport container has changed since the last shipment of the same product type was received for an unexplained reason (e.g., a notification about the change from the manufacturer has not been received).
To see if product inserts are missing, do not correspond to the product, or are suspicious in some way.
For shipping addresses, postmarks, or other materials indicating that the product came from an unexpected foreign entity or source.
Closely examine the label on the package, and the label on the individual retail unit, if applicable, for:
Any missing information, such as the lot number or other lot identification, NDC, or strength of the drug.
Any altered product information, such as smudged print or print that is very difficult to read.
Misspelled words.
Bubbling in the surface of a label.
Lack of an “Rx only” symbol6
Foreign language with little or no English provided.7
Foreign language that is used to describe the lot number.8
A product name that differs from the name that appears on the FDA-approved drug label or labeling.
A product name that is the product name for a foreign version of the drug.
A product that is transported in a case or tote, when not expected under the circumstances.
Lot numbers and expiration dates on product that do not match the lot numbers and expiration dates of its outer container.
Again, under section 582 of the FD&C Act, trading partners must have systems in place that enable them, upon determining that a product in their possession or control is suspect or upon receiving a request for verification from the FDA that has made a determination that a product within the possession or control of the trading partner is a suspect product, to quarantine suspect product and promptly conduct an investigation, in coordination with other trading partners, as applicable, to determine whether a suspect product is illegitimate. In addition, trading partners must, as applicable, make the notifications described in sections 582(b)(4)(B)(ii)(I), (c)(4)(B)(ii), (d)(4)(B)(ii), and (e)(4)(B) of the FD&C Act related to illegitimate product determinations, and, for manufacturers, the notification of a high risk of illegitimacy described in section 582(b)(4)(B)(ii)(II).
For Manufacturers: High Risk of Illegitimacy Notifications9
Section 582(b)(4)(B)(ii)(II) of the FD&C Act requires manufacturers to make notifications in certain circumstances for products that pose a high risk of illegitimacy. The provision states as follows:
(II) High risk of illegitimacy.--A manufacturer shall notify the Secretary and immediate trading partners that the manufacturer has reason to believe may have in the trading partner’s possession a product manufactured by, or purported to be a product manufactured by, the manufacturer not later than 24 hours after determining or being notified by the Secretary or a trading partner that there is a high risk that such product is an illegitimate product. For purposes of this subclause, a ‘high risk’ may include a specific high risk that could increase the likelihood that illegitimate product will enter the pharmaceutical distribution supply chain and other high risks as determined by the Secretary in guidance pursuant to subsection (h).
FDA interprets this provision to require manufacturers to notify [1] FDA and [2] the manufacturer’s immediate trading partners (that the manufacturer has reason to believe may have in the trading partner’s possession a product manufactured by, or purported to be a product manufactured by, the manufacturer) in three general scenarios:
Within 24 hours after determining or being notified by FDA or a trading partner that there is a high risk that a product that the manufacturer has reason to believe is in an immediate trading partner’s possession is an illegitimate product.
Within 24 hours after determining or being notified by FDA or a trading partner that there is a specific high risk that could increase the likelihood that illegitimate product will enter the U.S. pharmaceutical distribution supply chain.
Within 24 hours after determining or being notified by FDA or a trading partner that there exists an “other high risk” as determined by FDA in guidance pursuant to subsection 582(h).
FDA believes that Congress intended section 582(b)(4)(B)(ii)(II) to leverage the surveillance systems that many manufacturers already have in place to detect counterfeit and otherwise violative versions of their products. Manufacturers could learn about products with a high risk of illegitimacy from a variety of sources, including from within their own company, from their trading partners, from the FDA, or from other domestic and/or foreign regulatory authorities—even when a product may not be in the manufacturer’s possession or control.
Below are scenarios and examples in which a manufacturer should make a notification under section 582(b)(4)(B)(ii)(II).
1. High Risk of Illegitimacy Notification for Products that the Manufacturer Has Reason to Believe Are in an Immediate Trading Partner’s Possession
The first general scenario, described above, involves notifications for products that the manufacturer has reason to believe are in an immediate trading partner’s possession.
An example of this scenario might occur when the manufacturer is asked to coordinate a suspect product investigation by an immediate trading partner under section 582(c)(4)(B), 582(d)(4)(B), or 582(e)(4)(B), and the manufacturer determines that there is a high risk that the product is illegitimate. Some sample scenarios involving high risks of illegitimacy, where a manufacturer should make a notification, include:
A manufacturer learns from a trading partner that a suspect product purporting to be one produced by that manufacturer has been found in the U.S. pharmaceutical distribution supply chain. The manufacturer examines the suspect product and believes the product could be illegitimate but wants to take additional steps before determining that it is illegitimate. The manufacturer has reason to believe that additional illegitimate products are in the possession of immediate trading partners. For example, a wholesale distributor informs a manufacturer that it believes it has a counterfeit of that manufacturer’s product. The wholesale distributor sends the product to the manufacturer. The manufacturer examines the product and believes it could be counterfeit, but wants to perform a laboratory or other analysis for confirmation.
A manufacturer learns that its product has been stolen or diverted in the U.S. while not in its possession or control, and the manufacturer has reason to believe that an immediate trading partner might have the stolen or diverted product in its possession.
2. Specific High Risks That Could Increase the Likelihood of an Illegitimate Product Entering the U.S. Pharmaceutical Distribution Supply Chain
Section 582(b)(4)(B)(ii)(II) states that a high risk of illegitimacy may include a “specific high risk” that could increase the likelihood that illegitimate product will enter the pharmaceutical distribution supply chain. In such cases, the product has not yet entered the pharmaceutical distribution supply chain, so no immediate trading partners would have it in their possession. Section 582(b)(4)(B)(ii)(II) thus would require the manufacturer to make a notification to FDA, but the manufacturer would not be required to notify immediate trading partners. To help ensure the integrity of the supply chain, however, FDA recommends that a manufacturer notify its immediate trading partners of such “specific high risk[s]” even if that manufacturer does not have reason to believe that its immediate trading partners may have the high risk product in their possession. Some factual examples involving specific high risks include:
A manufacturer learns that a product with a high risk of illegitimacy (purporting to be one produced by that manufacturer) has been found in another country, and that such product is likely destined for a trading partner in the U.S. For example, the manufacturer learns from a foreign regulatory authority that one of its products has been counterfeited in another country, and that some of that product is on a cargo ship destined for the U.S. for delivery to a wholesale distributor.
A manufacturer learns that its product was stolen or diverted in another country, and that such product is destined for the U.S. in a manner that leads the manufacturer to believe the product will likely enter the U.S. pharmaceutical distribution supply chain. For example, the manufacturer learns from a foreign law enforcement agency that its product was stolen during transport in another country and is on a plane destined for the U.S. for delivery to a dispenser.
A manufacturer learns that there is a high risk that its product has been intentionally adulterated in another country such that the product would result in serious adverse health consequences or death to humans, and that such product is likely destined for the U.S. in a manner that leads the manufacturer to believe the product will enter the pharmaceutical distribution supply chain. For example, the manufacturer learns from its own investigation that there is a high risk that a contaminant that would result in serious adverse health consequences or death to humans was added to a product in another country and sent to a repackager in the U.S.
As noted above, these examples in sections 1 and 2 are examples, rather than an exhaustive list of circumstances in which trading partners should make notifications under section 582(b)(4)(B)(ii)(II).
3. Other High Risks as Determined by FDA: High Risk of Illegitimacy Notification Where a Manufacturer has Reason to Believe the Product has Entered the Pharmaceutical Distribution Supply Chain
Section 582(b)(4)(B)(ii)(II) of the FD&C Act permits FDA to determine, through guidance pursuant to section 582(h), “other high risks” that would trigger a notification under this provision. FDA believes that one “other high risk” not covered by the two general scenarios described above is when a manufacturer has reason to believe that an illegitimate product has entered the pharmaceutical distribution supply chain, even though the manufacturer does not have reason to believe that an immediate trading partner possesses the high risk product.10 As with the second general scenario, described above, section 582(b)(4)(B)(ii)(II) would require the manufacturer to make a notification to FDA, but the manufacturer would not be required to notify immediate trading partners. To help ensure the integrity of the supply chain, however, FDA recommends that a manufacturer notify its immediate trading partners of this “other high risk,” even if that manufacturer does not have reason to believe that its immediate trading partners may have the high risk product in their possession.
A manufacturer could learn that a product with a high risk of illegitimacy that was manufactured by or purported to be manufactured by that manufacturer may be in the possession of a trading partner, but that trading partner is not an immediate trading partner of the manufacturer. Some examples which involve this other high risk:
A manufacturer learns that a licensed health care practitioner is administering an oncology drug to patients that purports to have been manufactured by that manufacturer but the manufacturer determines that there is a high risk that the drug is a counterfeit. The licensed health care practitioner purchased the drug from a wholesale distributor, so he/she is not an immediate trading partner of the manufacturer. However, the manufacturer believes that the product has entered the pharmaceutical distribution supply chain.
A manufacturer learns that its product has been stolen or diverted in the U.S., and the manufacturer learns that a patient filled a prescription and received some of the stolen or diverted product. The patient suffers an adverse event and FDA and the manufacturer is notified of that situation. Because the dispenser did not purchase the product from the manufacturer, it is not an immediate trading partner of the manufacturer. However, the product has entered the pharmaceutical distribution supply chain.
A manufacturer learns that wholesale distributor B received product and transaction history going back to the manufacturer from wholesale distributor A, but the listed dosage form of the product on the transaction history is not one that has ever been used by the manufacturer. Wholesale distributor B provided a copy of the transaction history it received from wholesale distributor A to the manufacturer, and the manufacturer concluded after reviewing the copy and receiving similar reports from other trading partners that a fraudulent transaction had occurred. Because wholesale distributor B did not purchase the product from the manufacturer, it is not an immediate trading partner of the manufacturer. However, the product has entered the pharmaceutical distribution supply chain.
IV. Notification OF ILLEGITIMATE PRODUCT AND PRODUCTS WITH A HIGH RISK OF ILLEGITIMACY
As discussed above, trading partners must, as applicable, make the notifications described in sections 582(b)(4)(B)(ii)(I), (c)(4)(B)(ii), (d)(4)(B)(ii), and (e)(4)(B)(ii) of the FD&C Act related to illegitimate product determinations, and, for manufacturers, the notification of a high risk of illegitimacy described in section 582(b)(4)(B)(ii)(II). This section of the guidance addresses the process by which trading partners should notify FDA and trading partners regarding illegitimate products under section 582. After review of the circumstances surrounding the event, if FDA determines that notification is not required under either section 582(b)(4)(B)(ii)(I), (c)(4)(B)(ii), (d)(4)(B)(ii), (e)(4)(B)(ii) or (b)(4)(B)(ii)(II) of the FD&C Act, FDA intends to inform the submitting entity.
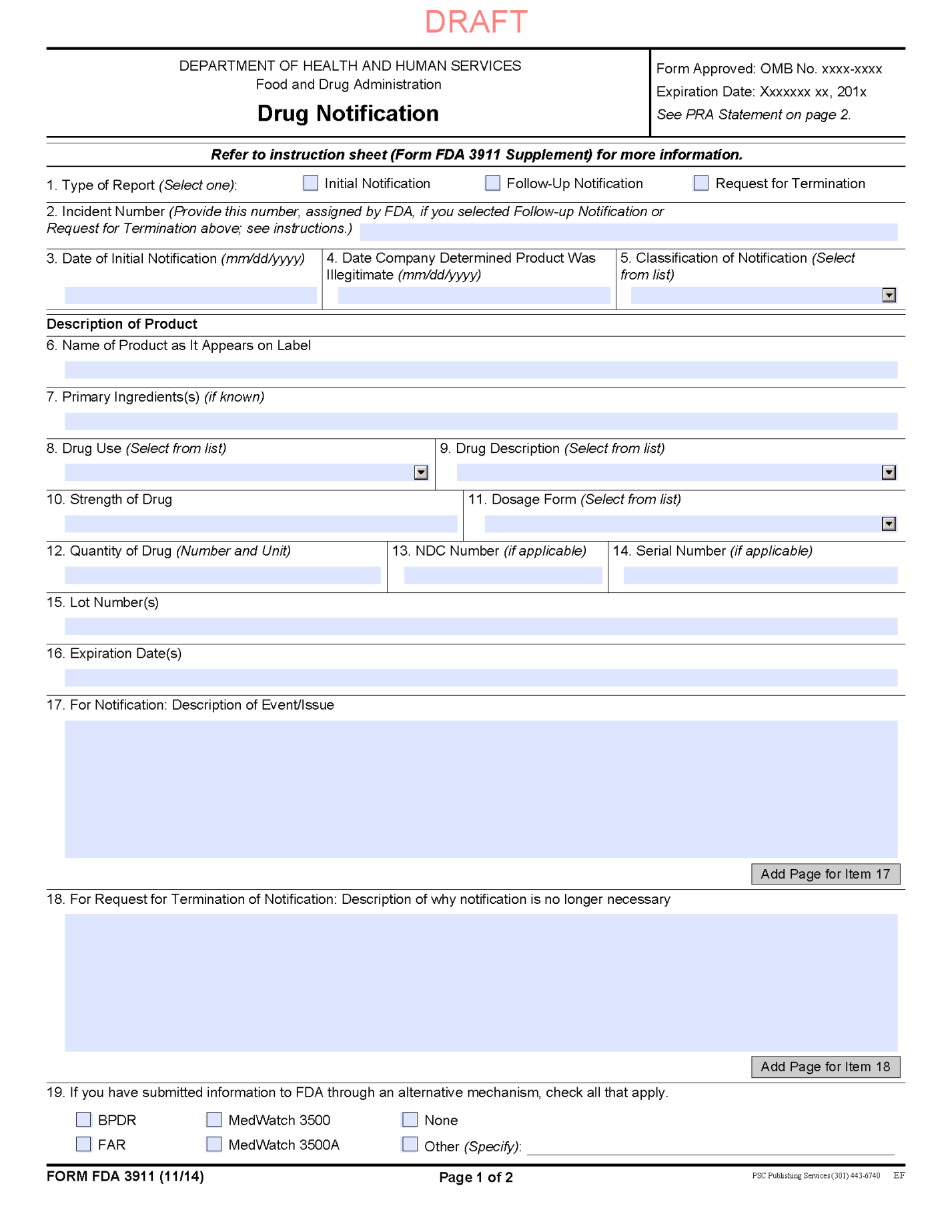
Trading partners should access FDA’s Web page at http://www.accessdata.fda.gov/scripts/cder/email/drugnotification.cfm for notifications.
Trading partners should follow the instructions on the Web page for accessing Form FDA 3911 (Attachment A). Using this form, trading partners should provide information about the person or entity initiating the notification, the product determined to be illegitimate that is the subject of the notification to FDA, and a description of the circumstances surrounding the event that prompted the notification.
Form FDA 3911 should be submitted using the method provided in the form or on the Web page.
FDA will acknowledge receipt of the notification and assign an incident number. This number should be referenced in all future correspondence about the illegitimate product including any request for termination.
In addition to notifying FDA, the trading partner that determines that it has an illegitimate product in its possession or control must notify all immediate trading partners that it has reason to believe may also possess the drug. Trading partners may notify other trading partners of an illegitimate product using existing systems and processes used for similar types of communications to those partners, which might include, but are not limited to, posting of notifications on a company Web site, telephoning, sending an email, or mailing or faxing a notification.
The following process should be used by manufacturers to notify FDA of a product with a high risk of illegitimacy:
To notify FDA of a product with a high risk of illegitimacy under section 582(b)(4)(B)(ii)(II), manufacturers should follow the instructions on the Web page for accessing Form FDA 3911 (Attachment A). Using this form, manufacturers should provide information about the person or entity initiating the notification, the product determined to have a high risk of illegitimacy that is the subject of the notification to FDA, and a description of the circumstances surrounding the event that prompted the notification.
FDA will acknowledge receipt of the notification and assign an incident number. This number should be documented in all future correspondence about the product with the high risk of illegitimacy including the request for termination.
In addition to notifying FDA, the manufacturer that determines that a product has a high risk of illegitimacy must notify all immediate trading partners that it believes may possess the drug. Manufacturers may notify trading partners of a product with a high risk of illegitimacy using existing systems and processes used for similar types of communications to those partners, which might include, but are not limited to, posting of notifications on a company Web site, telephoning, sending an email, or mailing or faxing a notification.
If a product with a high risk of illegitimacy is found to be an illegitimate product, manufacturers should submit a follow-up notification that explains the updated classification and references the incident number of the original notification of high risk of illegitimacy.
If it is determined that a product that was subject to a high risk of illegitimacy notification is not an illegitimate product, manufacturers must submit a request for termination of the high risk of illegitimacy notification to the FDA according to the process in Section B below.
Termination of Notification in Consultation With FDA11
Section 582(h)(2)(A) of the FD&C Act directs
FDA to issue guidance setting forth the process that trading partners
shall follow for terminating notifications regarding illegitimate
product, or for manufacturers, terminating notification of a high
risk of illegitimacy, in consultation with FDA, under section
582(b)(4)(B), (c)(4)(B), (d)(4)(B), and (e)(4)(B). Section
582(b)(4)(B), (c)(4)(B), (d)(4)(B), and (e)(4)(B) require trading
partners to have in place systems to enable them to terminate
notifications, in consultation with FDA, when appropriate. This
section of the guidance addresses the process by which trading
partners must terminate such notifications in consultation with FDA.
This process must be used when trading partners believe that a
notification they made to FDA regarding illegitimate product, or for
a manufacturer, a notification of a high risk of illegitimacy, is no
longer necessary.
The process for terminating notifications in consultation with FDA is as follows:
The trading partner making a notification to the FDA shall be responsible for making the request for termination.
Trading partners must access FDA’s Web page at http://www.accessdata.fda.gov/scripts/cder/email/drugnotification.cfm for termination of notifications.
Trading partners must follow the instructions on the Web page for accessing Form FDA 3911 (Attachment A). Using this form, trading partners must provide to FDA information about the person or entity initiating the request for termination, the illegitimate product or the product with a high risk of illegitimacy, the notification that was issued, and an explanation about what actions have taken place or what information has become available that make the notification no longer necessary. Trading partners should include the FDA-assigned incident number associated with the notification on the request for termination.
This form must be submitted by using the method provided in the form or on the Web page. The trading partner’s submission of a request for termination of a notification will be viewed as a request for consultation with FDA, as required in section 582 of the FD&C Act. FDA may request additional information it determines necessary to complete the consultation.
FDA will review the request and consult with the trading partner. The response time will depend on the number of requests for termination and the circumstances surrounding the requests for termination that are received by FDA.
FDA interprets the DSCSA’s requirement for trading partners to “mak[e] a determination, in consultation with the Secretary, that a notification is no longer necessary”12 to require that trading partners provide the Agency with an opportunity to provide its expert views and advice on proposed terminations of notifications. Therefore, a trading partner must wait until FDA responds to the termination request before the trading partner notifies other trading partners that a notification is terminated. FDA intends to respond to requests for termination within 10 business days of submission. In some cases, FDA may contact a trading partner to notify the partner that additional time is needed to respond to the request for termination. If a trading partner believes that exigent circumstances require expedited consideration of a termination request (e.g., a potential drug shortage), the trading partner must describe those circumstances in the termination request to FDA on the FDA Form 3911 when making the request for termination.
Under section 582(b)(4)(B), (c)(4)(B), (d)(4)(B), and (e)(4)(B) of the FD&C Act, after FDA provides its consultation response, and the trading partner determines that the notification is no longer necessary, the trading partner that made the request for termination must promptly notify immediate trading partners that the notification has been terminated. Trading partners may notify their trading partners of a termination using existing systems and processes used for similar types of communications to those partners, which might include, but are not limited to, posting of notifications on a company Web site, telephoning, sending an email, or mailing or faxing a letter or notification.
ATTACHMENT A: FORM FDA 3911




1 This guidance has been prepared by the Office of Compliance in the Center for Drug Evaluation and Research (CDER) in cooperation with the Center for Biologics Evaluation and Research (CBER) and the Office of Regulatory Affairs (ORA) at the Food and Drug Administration.
2 Congress gave FDA authority to implement through binding guidance the process for terminating notifications of illegitimate product in consultation with FDA. Thus, the discussion of the termination process in section IV has binding effect.
3 For this guidance, trading partner is defined as described in section 581(23)(A) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 30eee(23)(A)), and refers to a manufacturer, repackager, wholesale distributor, or dispenser. For purposes of this guidance, trading partner does not refer to a third-party logistics provider (3PL) as defined at section 581(23)(B) of the FD&C Act (21 U.S.C. 360eee(23)(B)), though FDA encourages 3PLs to follow the recommendations in this guidance to the extent relevant to the 3PL’s operations.
4 Section 582 of the FD&C Act gives FDA authority to issue binding guidance on the process for terminating notifications of illegitimate product. Specifically, section 582(h)(2)(A) states that FDA “shall issue a guidance document to aid trading partners in the identification of a suspect product and notification termination. Such guidance document shall . . . set forth the process by which manufacturers, repackagers, wholesale distributors, and dispensers shall terminate notifications in consultation with the Secretary regarding illegitimate product . . . .”
5 For additional definitions applicable to this guidance, please refer to section 581 of the FD&C Act.
6 Or, for products distributed solely in the Commonwealth of Puerto Rico or in a Territory where the predominant language is Spanish, “Solamente Rx”. 21 CFR 201.16
7 Except for products distributed solely in the Commonwealth of Puerto Rico or in a Territory where the predominant language is one other than English. 21 CFR 201.15 (c)(1)
8 Except for products distributed solely in the Commonwealth of Puerto Rico or in a Territory where the predominant language is one other than English. Id.
9 This section of the guidance is being distributed for comment purposes only.
10 FDA reserves authority to articulate additional “other high risk[s]” in subsequent guidance.
11 As described above, this section of the guidance document is binding.
12 FD&C Act § 582(b)(4)(iv), (c)(4)(B)(iv), (d)(4)(B)(iv), and (e)(4)(B)(iv).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy