HCHS SOL Supporting Statement B FINAL May2014
HCHS SOL Supporting Statement B FINAL May2014.docx
The Hispanic Community Health Study/ Study of Latinos (HCHS/SOL)(NHLBI)
OMB: 0925-0584
The Hispanic Community Health Study/ Study of Latinos (HCHS/SOL)
Supporting Statement Part B
January 2014
OMB#: 0925-0584
Contact: Larissa Avilés-Santa, MD, MPH
National Institutes of Health
National Heart, Lung and Blood Institute
(NIH/NHLBI)
6701 Rockledge Drive MSC 7936
Bethesda, MD 20892
Phone: 301-435-0450
FAX: 301-480-1455
E-mail: avilessantal@nhlbi.nih.gov
B. Collections of Information Employing Statistical Methods
B.1. Respondent Universe and Sampling Methods
B.1.a. Design Summary
The sampling and recruitment plan for the HCHS/SOL was designed to support four analysis objectives. To accomplish these objectives, a representative sample of participants in the target areas at each field center was selected. Methods of sample selection, recruitment, and retention were designed to maximize participation rates, minimize non-response, and minimize attrition during the follow-up period. These methods were explained in the original application, and have been published [Sorlie et al, and LaVange et al in Appendix 1].
First, the HCHS/SOL sample supports estimates of the prevalence (or mean values) of baseline risk factors for 1) all Hispanics combined in this study; 2) all study Hispanics by community of residence; 3) all study Hispanics by country of origin; 4) to a limited extent all study Hispanics by community of residence controlling for country of origin; and 5) to a limited extent all study Hispanics by country of origin controlling for community of residence. Secondly, the study sample supports evaluation of the relationships between the various risk factors, demographic factors, and cultural factors collected at baseline. Thirdly, the study sample supports evaluation of factors collected at baseline in relation to the incidence of disease and death that will occur during the follow-up period. During the previous follow-up period, the number of disease events was small (under 100) due to the relatively young age structure of this Hispanic cohort and length of follow-up. However, within the proposed next follow-up period, there will be a larger number of events that accrue overall (approximately 266 CVD events and about 230 asthma/COPD hospitalizations and over 500 pregnancy related admissions) for a broad analysis of risk factors and incidence of cardiovascular and pulmonary disease outcomes.
The table below presents the actual counts for the first occurrence of an event reported from 2008 to 2011 and an estimated rate per 1,000 person years of follow-up. Cohort participants with the prevalent disease at baseline are excluded from the projections. For these preliminary estimates events are classified according to ICD-9 coded discharge diagnoses abstracted from the medical records by HCHS-SOL field center personnel. Classification of events per the HCHS-SOL protocol and event adjudication has not yet been applied to the events accrued through 2011. Projected numbers and rates for 2012 to 2019 are presented assuming and average 6 additional years of follow-up on all AFU eligible cohort members. Projected number of CVD deaths would be approximately 156 based on rates for Hispanics recently published in Circulation (Note: projections of events and rate counts are not adjusted for the aging of the cohort. The actual validated number of events accruing over time may be different as the presented counts are based on ICD codes only).
Projected First Events in HCHS/SOL, and 2012 to 2019 Projections in the HCHS/SOL Cohort. Based on ICD Coded Hospital Discharge Diagnoses
ICD-9 Event Code Categories |
|
2008 to 2011 |
|
2012 to 2019 |
Total by 2019 |
|||
|
|
Observed First events |
Estimated Rate/1000 person-years |
|
Projected Number First events |
All Events Observed And Projected |
|
|
Asthma/COPD |
|
51 |
1.78 |
|
230 |
281 |
|
|
Myocardial Infarct |
|
20 |
0.70 |
|
90 |
110 |
|
|
Heart failure |
|
37 |
1.29 |
|
167 |
204 |
|
|
Stroke |
|
32 |
1.16 |
|
144 |
176 |
|
|
Pregnancy related |
|
116 |
6.73 |
|
519 |
635 |
|
|
|
|
|
|
|
|
|
|
|
Deaths - All Causes |
|
98 |
3.42 |
|
441 |
539 |
|
|
Note: The above event counts are based on from ICD-9 codes in the medical record.
Asthma/COPD: Pulmonary Codes 491, 492, 493, 496
Myocardial Infarction: MI code 410
Heart Failure: HF Code 428
Stroke: Stroke Codes 430 to 434
Pregnancy: Pregnancy Complication Codes 641 to 679
The re-examination of these cohorts proposed in this application, will provide estimates of factors related to change in the measured characteristics, and the ability to estimate their impact on disease and death.
B.1.b. Statistical Considerations and Power
The following table describes the actual sample size at the baseline examination by country of origin and community.
Table B.1 Number of HCHS/SOL Baseline Examination Participants
Community |
Country of Origin |
|||||||
Dominican |
Central American |
Cuban |
Mexican |
Puerto Rican |
South American |
Mixed/Other |
Total |
|
Bronx |
1380 |
219 |
45 |
208 |
1837 |
187 |
200 |
4076 |
Chicago |
27 |
418 |
25 |
2409 |
770 |
374 |
100 |
4123 |
Miami |
64 |
1034 |
2269 |
38 |
82 |
468 |
112 |
4067 |
San Diego |
2 |
61 |
9 |
3817 |
39 |
43 |
91 |
4062 |
Total |
1473 |
1732 |
2348 |
6472 |
2728 |
1072 |
503 |
16328 |
Frequency Missing = 87 Shaded boxes indicate adequate sample size for comparisons between background and sites. |
||||||||
As can be seen from this table, the final number of enrolled participants for each community at baseline is large (over 4000 each), and it also is large for each country of origin group (excess of 1000 in each group). These sample sizes in this table are appropriate for comparisons by community, or comparisons by country of origin (sub-objectives 2 and 3 above). For sub-objectives 4 and 5 above, however, analyses are more limited. Because of the geographical clustering by country of origin, there is not complete diversity of origin for all communities. Groups of 150 or more can produce estimates of mean values with sufficiently high statistical power so that comparisons for some of the community and ethnic group combinations can be made. For example, within the Bronx and Chicago, reasonable comparisons can be made among all groups, except Cubans. Because San Diego is nearly all Mexican American, it is the only community that will not have within-community comparisons by country of origin.
The second analytical goal was to assess the relationships among the variables collected at baseline. These analyses are nearly unlimited in number, but include comparisons of risk factors and other measured characteristics. A list of the types of analyses would take many pages, but examples of some analyses would include evaluation of the relationship between body mass index and blood pressure or cholesterol; the relationship between hypertension and length of residence in the U.S.; the relationship between dietary factors of fat and carbohydrates with obesity or lipids; the relationship between perceived Hispanic identity and use of health care services; the relationships between hearing loss and occupations that involve machine noises; the relationships between sleep quality and metabolic syndrome; the relationship between daily physical activity and diabetes or obesity. Sample size/power analysis will be presented for comparative examples in the Sample Size/Power Analysis section below.
The third analytical goal is to assess the relationship between baseline characteristics and incident disease and death. As indicated, the first 3½ years of follow-up that took place during the first funding period yielded a very small number of cardiovascular and pulmonary events to generate a sufficiently powered analysis. The proposed extended follow-up (an average of 6 additional years) will add to the accrual of events.
The final analytical goal is to assess change in characteristics since enrollment. The proposed re-examination (Visit 2) will provide the opportunity to reassess select cardiovascular risk factors and other variables, and describe the mediators of change in risk and development of overt disease. Since the baseline findings demonstrate that the prevalence of cardiovascular risk factors among this cohort of diverse Hispanic groups of origin is widespread and number of risk factors varies according to Hispanic group, the reassessment of risk factors will provide a unique opportunity to objectively measure changes over time, and statistically powered assessment of risk.
Sample Size/Power Analysis
The following table (Table B.2.) presents the estimated number of participants projected to attend Visit 2 based on an assumption that a minimum of 85% of the participants will return for a re-examination.
Table B.2 . Estimated Number of HCHS/SOL Visit 2 Examination Participants
Community |
Country of Origin |
|||||||
Dominican |
Central American |
Cuban |
Mexican |
Puerto Rican |
South American |
Mixed/Other |
Total |
|
Bronx |
1173 |
186 |
38 |
177 |
1561 |
159 |
170 |
3464 |
Chicago |
23 |
355 |
21 |
2048 |
654 |
318 |
85 |
3504 |
Miami |
54 |
879 |
1929 |
32 |
70 |
398 |
95 |
3457 |
San Diego |
2 |
52 |
8 |
3244 |
33 |
37 |
77 |
3453 |
Total |
1252 |
1472 |
1996 |
5501 |
2318 |
912 |
427 |
13878 |
Assumes 85% of participants are re-examined at visit 2 Shaded boxes indicate adequate sample size for comparisons between background and sites. |
||||||||
Statistical power calculations are shown below for a variety of examples. Because the cohort has been assembled, we take the approach of presenting the minimum detectable odds ratios, mean differences, or hazard ratio at 80% power with estimated sample sizes. We conducted power analysis for cross-sectional associations, longitudinal analyses, and time-to-event (incidence) analyses. For cross-sectional analyses of characteristics associated with binary outcomes (e.g., asthma), Table B.3 shows minimum odds ratios (ORs) that can be detected at 80% power using the entire cohort (n is approximately= 14,000) or Hispanic/Latino groups (n is approximately 8,000 or 3,000). Assuming that the prevalence of the outcome is 10%, we varied the relative number of subjects in the exposed versus unexposed groups. A 1:1 ratio for the low-risk versus high-risk group corresponds to a continuous exposure variable dichotomized at its median; a 3:1 ratio corresponds to a relatively common risk factor (25%) or to comparing the top quartile of a continuous exposure variable to the lower three quartiles; and a 10:1 ratio corresponds to a less frequent risk factor (e.g. pre-eclampsia). The column identified as variance inflation factor (VIF)=1 provides the minimum detectable ORs without considering confounding or a design effect (i.e. no variance inflation from the study design); these are not applicable because of the two-stage sampling design and are displayed as a lower bound for the minimum detectable ORs. Based on our experience with the HCHS/SOL data, the VIF can vary from 1.5 to 5. Hence we provide minimum detectable ORs when VIF=2 or 5. When the ratio of subjects in the two groups is 1:1, the minimum detectable odds ratios using the entire cohort (n=14000) with 80% power are generally lower than 1.5, and lower than 1.8 for the other situations. For example, with VIF=2 and assuming the prevalence of CHD is 10%, the minimum detectable OR for comparing the high to low SES groups (divided at the median) is 1.25.
Table B.3. Minimum detectable prevalence odds ratios with 80% power |
||||||||||
Outcome prevalence in low-risk group |
Total sample size |
VIF=1 |
VIF=2 |
VIF=5 |
||||||
Ratio of subjects in low risk: high risk groups |
Ratio of subjects in low risk: high risk groups |
Ratio of subjects in low risk: high risk groups |
||||||||
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
||
10% |
14000 |
1.17 |
1.20 |
1.31 |
1.25 |
1.29 |
1.45 |
1.41 |
1.48 |
1.77 |
8000 |
1.23 |
1.27 |
1.42 |
1.33 |
1.39 |
1.62 |
1.56 |
1.66 |
2.09 |
|
(e.g., cardiac dysfunction) |
3000 |
1.39 |
1.46 |
1.74 |
1.58 |
1.68 |
2.14 |
2.01 |
2.21 |
3.15 |
|
|
|
|
|
|
|
|
|
|
|
For continuous outcomes such as change in risk factors between visits 1 & 2, Table B.4 shows the standardized mean differences that can be detected with 80% power for various combinations of sample size, relative number of subjects in the groups, and VIF. For example, with VIF=2, the minimum detectable mean difference in the change in risk factors over an average of 6 years between the Dominicans and Central Americans (around 1500 participants per group) is 0.15SD, where SD is the standard deviation of the change. If the SD for BMI, waist circumference, systolic BP, and glucose is 2.06, 6.56, 15.13, and 24.76, respectively, then the minimum detectable differences comparing Dominicans and Central Americans for the change in BMI, waist circumference, systolic BP, and glucose is 0.31 kg/m2, 0.98 cm, 2.27 mmHg, and 3.71 mg/dL, respectively.
Table B.4. Minimum detectable standardized mean difference with 80% power |
||||||||||
Total Sample Size |
VIF=1 Ratio of subjects in low risk: high risk groups |
VIF=2 Ratio of subjects in low risk: high risk groups |
VIF=5 Ratio of subjects in low risk: high risk groups |
|||||||
|
|
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
14,000 |
0.05 |
0.06 |
0.09 |
0.07 |
0.08 |
0.12 |
0.11 |
0.13 |
0.19 |
|
8,000 |
0.07 |
0.08 |
0.11 |
0.09 |
0.11 |
0.16 |
0.15 |
0.17 |
0.25 |
|
3,000 |
0.11 |
0.12 |
0.18 |
0.15 |
0.17 |
0.26 |
0.23 |
0.27 |
0.41 |
|
|
|
|
|
|
|
|
|
|
|
|
Power calculations for the study of relationships between exposures and incident events (e.g. CHD, stroke) are based on the estimated incidence of events defined by ICD-coded hospital discharge diagnoses. Table B.5 shows the minimum hazard ratios that can be detected for various subgroup sample sizes assuming the incidence rates range from 2 (e.g., hospitalized MI) to 10 (e.g. onset of diabetes) per 1,000 person years with 9.5 years total follow-up. The minimum detectable hazard ratio ranges from 1.63 to 4.79 when the incidence rate is 2 events per 1,000 person years, and range from 1.27 to 2.62 when the incidence rate is 10 events per 1,000 person years.
Table B.5. Minimum detectable hazard ratio with 80% power |
||||||||||
Incidence in low-risk group |
Total sample size |
VIF=1 Ratio of subjects in low risk: high risk groups |
VIF=2 Ratio of subjects in low risk: high risk groups |
VIF=5 Ratio of subjects in low risk: high risk groups |
||||||
|
|
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
1:1 |
3:1 |
10:1 |
|
14000 |
1.43 |
1.47 |
1.69 |
1.63 |
1.68 |
1.99 |
2.07 |
2.13 |
2.61 |
2 /1,000 person years |
8000 |
1.56 |
1.62 |
1.90 |
1.83 |
1.90 |
2.29 |
2.45 |
2.50 |
3.11 |
(e.g. MI) |
3000 |
2.02 |
2.08 |
2.55 |
2.57 |
2.61 |
3.26 |
3.91 |
3.80 |
4.79 |
|
|
|
|
|
|
|
|
|
|
|
|
14000 |
1.19 |
1.21 |
1.31 |
1.27 |
1.30 |
1.44 |
1.44 |
1.48 |
1.71 |
10/1,000 person years |
8000 |
1.24 |
1.27 |
1.40 |
1.35 |
1.39 |
1.58 |
1.58 |
1.63 |
1.93 |
(e.g. Diabetes) |
3000 |
1.42 |
1.47 |
1.69 |
1.62 |
1.67 |
1.99 |
2.05 |
2.12 |
2.62 |
B.1.c. Sample Selection
Sample selection was accomplished through a multi-stage area probability sample implemented for each site [LaVange et al and Daviglus et al, Appendix 1]. At the first stage, a stratified sample of Census block groups was selected. Stratification factors common across the four field centers were (1) low versus high SES (as measured by the proportion of persons with at least a high school education) and (2) low vs. high concentration of Hispanic/Latino households, resulting in four strata per field center. Selection of block groups was carried out proportionately with respect to the SES strata and disproportionately with respect to the Hispanic/Latino concentration strata, that is, block groups in the high concentration stratum were selected at a higher rate than those in the lower concentration stratum. This over-sampling was carried out to maximize efficiencies in the field by increasing the probability that a selected household is a Hispanic/Latino household. In addition to these four strata, block groups in the Coop City area were isolated into a 5th stratum in the Bronx, and block groups representing high concentration areas for Central and South Americans were isolated into a 5th stratum in Miami. Both of these ‘special’ strata were defined to ensure selection of adequate numbers of households in the respective areas.
At the second stage, households in the sampled block groups were selected from a dual frame constructed from non-over-lapping lists of postal addresses and Hispanic/Latino surnames. Addresses were selected from the surname list at a higher rate than from the postal list, to further maximize efficiency of field operations by increasing the probability that a selected household is a Hispanic/Latino household. Selected households were screened for eligibility, where eligibility is defined as at least one Hispanic/Latino household member aged 18-74 years. Eligible households in which all Hispanic/Latinos in the target age range were at least 45 years of age were selected with certainty (probability of selection = 1), while all other households were selected with probability (0 ≤ p < 1) based on the expected household composition for the area. Once a household was selected, all members of the household were invited to participate. This household selection algorithm was designed to provide the target age distribution for the HCHS/SOL study, namely, 62.5% of participants aged 45-74 years and 38.5% aged 18-44 years, and to minimize the amount of information required for screened households that might have not been selected for participation. Selection of households corresponded to an over-sampling of Hispanic/Latinos in the older age range, which was necessary given the age distribution of Hispanic/Latinos currently living in the U.S.
Recruitment was planned and took place over a three-year period. The sample of households in each target area was randomly allocated to each of the three years of recruitment. Within each recruitment year, the sample was fielded in waves, with each wave corresponding to a random sub-sample of the original sample of households allocated to that year.
B.1.d. Over four thousand (4,000) persons aged 18-74, who self-identified as Hispanic/Latino origin, independent of country of origin were selected from each of four separate communities.
Bronx: over 644,000 residents of Hispanic/Latino origin

For recruiting, areas of the Bronx that have the highest Hispanic/Latino concentration and that were in closest proximity to the Bronx Field Center location(s) in the South and East Bronx were targeted. The previous map above highlights the specific recruiting areas for the Bronx. Areas highlighted represent the selected census tracts
Chicago: over 780,000 residents of Hispanic/Latino origin


The targeted area for the Chicago site was composed of ethnically diverse neighborhoods with several that have been majority Hispanic/Latino for decades as well as others that were traditionally White/European-immigrant which have experienced Hispanic/Latino in-migration only recently. The highlighted areas in the map above represent the selected census tracts in the Cook County, where Chicago is located.
Miami/Dade County: over 1.3 million residents of Hispanic/Latino
origin
The highlighted areas of the previous map represent the selected census tracts in Miami-Dade County. This area consisted of approximately 25 contiguous census tracts beginning just south of the Miami Field Center and extending further south and west to the city of Coral Gables. The balance of the targeted census tracts was located in the city of Miami, while 14 additional census tracts were located in the city of Hialeah.
San Diego: almost 1 million residents of Hispanic/Latino origin
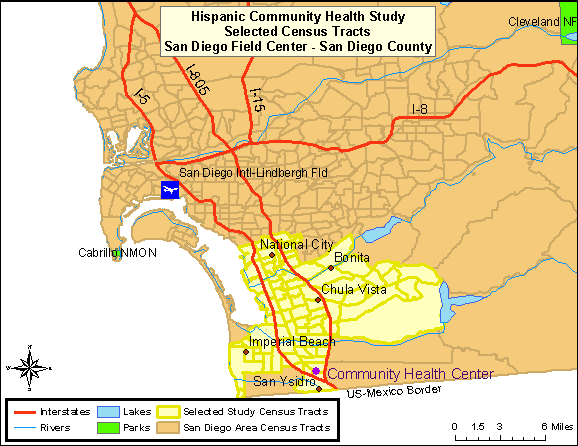

The combined region of South Suburban and South-Central San Diego County, commonly referred to as the “South Bay”, was the target community. This area included the communities of San Ysidro, Chula Vista, Imperial Beach, National City, and Bonita. These areas contained large proportions of minority residents, with Hispanics/Latinos representing the largest percentage [U.S. Census Bureau 2005]. The highlighted areas in Map 4 represent the selected census tracts in the San Diego County.
Recruitment was designed to occur in stable, established, communities so that persons could be contacted over time. Each community has a community social infrastructure and organization that enables community support and feedback.
B.1.e. Participants’ Call Back
The participants’ call back plan consists of three basic steps:
Initial mailings to participants describing the new visit (Visit 2) [Example of Bronx Newsletter, Appendix 17]
During the ongoing annual follow-up telephone interviews, participants will be informed of the new visit (Visit 2). Scheduling of the visit could take place during that call.
Home visits could also take place, when telephone contacts are not possible, or when participants request it.
The Field Centers have the option in the call back procedures to either make the first contact with a participant via a phone call, or as a home visit.
B.2. Procedures for Information Collection
Data collection for the HCHS/SOL requires questionnaires in each domain of measurement to be available in both English and Spanish versions. Trained, bilingual interviewers will administer the study questionnaires. Questionnaires for which no existing Spanish translations are available are translated by a subcontracting firm, Chicle, with expertise in multilingual instrument development for large-scale surveys. Both new and existing translations are then reviewed by the Translation Committee. The results of the reviews are discussed via teleconference, and a summary of recommended changes to the translation are sent back to Chicle for final review and certification.
The Translation Committee includes members from the four field centers, the coordinating center and the project office who are bilingual and native Spanish speakers and represent most of the countries represented in the cohort. They reviewed all the instruments and evaluated the reading level, the quality of the translations (grammatical quality and use of terms that are understood by Hispanics/Latinos of a diversity of origins), and the cultural relevance and appropriateness of the questions. This process of evaluation was not limited to existent versions in Spanish or translations done for the study. The English versions were evaluated as well. During this process, the committee identified some phrases or words that could have different interpretations or that needed some modification of their reading level. Hence, alternate definitions or idiomatic expressions have been incorporated into the interviews when appropriate. These are known as Question by Question instructions or “QxQs”. If a participant does not understand the meaning of a term, the interviewer will be able to view a help screen with the definitions or alternative term. In consultation with our medical investigators, medical terms need to remain in the questionnaires with appropriate explanations to the interviewers and participants [Appendix 19 - Example of QxQ].
B.2.b. Cohort Surveillance Component Design
The Study identifies, abstracts, reviews, and validates cardiovascular and pulmonary events (requiring emergency room visit or hospitalization, or based on death information) which occur in the interim between the baseline exam and each subsequent annual follow-up telephone call. Cardiovascular events include fatal and non-fatal myocardial infarction, sudden cardiac death, fatal and non-fatal stroke, and fatal and non-fatal heart failure. Pulmonary events include exacerbation of chronic obstructive lung disease and asthma. All-cause mortality has also been one of main endpoints of the surveillance. Preeclampsia, eclampsia and gestational diabetes mellitus are new endpoints that will be ascertained during the proposed study period. In more detail, we do the following:
Identify potential events from the AFU telephone call which provide information that a hospitalization or ER visit took place and the reason for the visit. Similarly, identify deaths from information obtained at the AFU telephone call and from a review of the vital statistics lists and obituaries from the state in which the community is located. The Coordinating Center (or Field Center if required for confidentiality) is responsible for conducting a match to the National Death Index periodically.
Request and obtain medical records of relevant events, abstract necessary information to validate the diagnoses, and enter it into the study database.
Review the abstracted information and validate the diagnoses using trained and certified clinicians designated from each Field Center (a morbidity and mortality classification committee).
Tabulate cause of death by obtaining, abstracting, and reviewing all relevant information from death certificates. This information will be confirmed by the next-of-kin, coroner, participant’s primary physician, nursing home and hospital records.
B.3. Methods to Maximize Retention Rates and Address Low Retention
B.3.a Retention Rates
HCHS/SOL study employed a probability sampling design. A stratified two-stage area probability sample of household addresses was selected from subjectively designated Hispanic neighborhoods defined by the set of census tracts serving as the sampling target population in each of the four field centers. After households were randomly selected, in-person visits and/or telephone contacts were made to screen eligible households and to roster its members. The household-level response rate was 33.5%. Of 39, 384 individuals who were selected, screened and met eligibility criteria, 41.7% were enrolled, representing 16 415 persons from 9872 households. Even though the response rate for the baseline examination was low, a widely accepted statistical adjustment protocol was followed to reduce the potential bias of estimates due to study non-participation. To minimize this bias effect while controlling the precision loss implications of adjustment, the sample weight of each participant was: (1) calculated based on its selection probability; (2) adjusted for differential non-response at the household and person levels, and trimmed to reduce the variability of the adjusted weights; and (3) calibrated to the 2010 Census count by age, gender, and Hispanic background in each field center’s target population. This three-step approach to calculate sample weights is consistent with weighting strategies used in all major health surveys utilizing probability sampling (e.g., NHANES, NHIS, and MEPS). Thus, as with other comparable population-based sample, to address the potential bias in the respondent sample, sample weights should be used in the analysis of this data.
B.3.b. Methods to Maximize Retention
A Cohort Retention Committee was created during the previous study period to assist in the development and implementation of a strategic plan to stay in touch with participants, learn of their vital status, engage them in completing the AFU interview, and track AFU interview completion. This committee is also developing a call back plan and strategies to engage participants in attending the Visit 2 examination, informing them of baseline findings (via a Participant Booklet, Appendix 17] and publications (via fact sheets, example included in Appendix 17] and what those findings mean to the Hispanic community. A Community Relations Committee was also created during the previous study period, which will develop plans to disseminate study findings to the local communities and inform the Hispanic community at large of the study activities, progress and publications. The Community Relations Committee activities may include making brief presentations about the project to community block clubs, local churches, and community-based organizations, among other strategies.
The trend in response rates over time for the first through fourth years of annual follow-up are summarized below from both current study progress monitoring reports. The first two years of annual interview contacts are complete so those response rates of 88 and 86% are final. The fourth year interviews have a completion rate of 84% based on reports from 81% of the expected interviews. However, since the cohort was recruited in 3 yearly waves the fourth and fifth years of interviews are ongoing and it is too early to project final response rates with 56% or more of interviews still outstanding at this time. The Cohort Retention Committee closely monitors these rates and is working on a continual basis with field centers to improve their response over time.
Table B.6. HCHS/SOL Projected Follow-up Response Rates
by Community and Year
Year of AFU interview1 |
Expected Percentage Contacted |
Bronx |
Chicago |
Miami |
San Diego |
Overall Centers |
First |
100.0 |
82.3 |
87.1 |
92.6 |
88.3 |
87.6 |
Second |
99.3 |
80.6 |
88.8 |
90.8 |
87.3 |
86.9 |
Third |
80.7 |
76.0 |
86.3 |
85.6 |
86.9 |
83.7 |
Fourth |
44.4 |
70.0 |
81.2 |
85.2 |
82.0 |
79.4 |
Fifth2 |
12.1 |
n/a |
n/a |
n/a |
n/a |
n/a |
1- First and second years of annual follow-up are closed but
Interviews from third through year onwards remains active
2- Fifth year of interviews has insufficient follow-up time for estimates
The Cohort Retention Committee has found that the use of alternate respondents tends to be highest in the Bronx and in San Diego for AFU years 1 and 2. The use of alternates is increasing in Miami in AFU year 3. This may reflect the transience of the populations. Higher response rates in Miami partially reflects their ability to make use of Social Security numbers in tracking down new contact information for participants who move. The overall Year 1 and Year 2 results reflect the same pattern found in AFU Year 3. Differences in contact and response rates were not found by country of origin. Despite challenges, response rates for HCHS are higher than response rates for Hispanics in other national studies and compare favorably with overall response rates in other biomedical studies such as ARIC.
Educational level and literacy were factors seriously considered during the development of all the instruments used in the study. It is important to emphasize that all questionnaires are administered verbally by trained interviewers in either English or Spanish, according to participants’ preferences. Interviewers will be able to repeat questions, and in the cases that merit it, participants will receive a card with the scales or alternative answers printed on them, to facilitate their understanding and obtain more accurate responses.
With permission of the participant, the interviews will be monitored for quality control purposes. Modifications will be made to questionnaires as needed based on experience with the interviews and these quality control checks. Any modifications to the questionnaires will be forwarded to OMB.
Most of the instruments to be used in the study have been used or adapted from other epidemiological studies and, therefore, have been previously validated in their current version. Therefore, for comparability, the language needs to remain consistent. One questionnaire in the sociocultural domain of family cohesion is under copyright.
Verification of eligibility for all study components is part of the Visit 2 scheduling procedure. Following an explanation of the HCHS/SOL study Visit 2 and the measurements involved, the interviewer will request an opportunity to verify the individual’s eligibility for all of them. The conditions reviewed during this interview (and listed on the form) include pregnancy, and the participant’s use of a pacemaker or other implanted electronic device. Study participants who are pregnant are asked to schedule an examination visit at three months after delivery, and to provide a date by which the HCHS/SOL can re-contact them for this purpose.
During this interview staff also inquires about special needs, such as any medical conditions that would affect the examination or the appointment time, difficulties in getting on or off an examination table, or impediments in hearing or reading. Arrangements for a safe and comfortable examination visit are made, consulting with the Clinic Manager as appropriate.
Throughout the call back period the Coordinating Center will prepare reports to monitor enrollment by center, gender, country of origin, and age. Scheduling and examination status reports will plot expected vs. actual recruitment rates for each Field Center, including cumulative and short-term performance. These reports will be available on the study web site to enable the Steering Committee and NHLBI staff, as well as Field Center personnel, to closely monitor overall recruitment.
The Study will hold periodic conference calls of the cohort Visit 2 recall and retention supervisors of each Field Center with the Cohort Retention Committee. Initially these calls will be held at least twice a month, until a Field Center’s visit attendance rate tracks at or above the projected re-examination goal. The purpose of the calls will be to review successful strategies and to facilitate the sharing of materials. A Field Center with sub-optimal return yield will be asked to develop supplemental or alternative techniques to improve Visit 2 recruitment rates. If a Field Center were to encounter persistent call back attendance difficulties, a monitoring visit will be made.
B.4. Test of Procedures or Methods to be Undertaken
There will be no new procedures or methods of data collection undertaken during the HCSH/SOL. The procedures and methods of data collection are proven and have been used in previous collections to minimize burden and improve utility. The attachment Sources of Questionnaires and Protocols [Appendix 20] provides the source of procedures and protocols used in this study.
B.5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
The following individuals were consulted on statistical aspects:
William Kalsbeek, Ph.D. Phone: (919) 962-3249
Professor of Biostatistics
Past Director, Survey Research Unit
University of North Carolina, Chapel Hill
Jianwen Cai, Ph.D. Phone: (919) 966-7788
Professor of Biostatistics and Vice Chair
Collaborative Studies Coordinating Center,
University of North Carolina, Chapel Hill
Donglin Zeng, Ph.D. Phone: (919) 966-7273
Professor of Biostatistics, Co-director Carolina Survey Research Laboratory
University of North Carolina, Chapel Hill
The following individuals are responsible for data collection:
Jianwen Cai, PhD Phone: (919) 966-7788
Collaborative
Studies Coordinating Center
University of North Carolina, Chapel Hill
Martha Daviglus, MD, PhD Phone: (312) 413-0739
Chicago Field Center: University of Illinois at Chicago
Neil Schneiderman, PhD Phone: 305-284-5467
Miami Field Center: University of Miami
Gregory A. Talavera, MD, MPH Phone: 619 594-4086
San Diego Field Center: San Diego State University
Robert Kaplan, PhD Phone: (718) 430-4076
Bronx Field Center: Albert Einstein College of Medicine
Scott Solomon, MD Phone: (857) 307-1960
Echocardiography Reading Center: Brigham and Women’s Hospital
The following individuals are responsible for data analysis:
Jianwen Cai, PhD Phone: (919) 966-7788
Collaborative
Studies Coordinating Center
Department of
Biostatistics
University of North Carolina at Chapel Hill
Gerardo Heiss, M.D., Ph.D. Phone: (919) 962-3253
Department of Epidemiology
Collaborative Studies Coordinating Center
University of North Carolina, Chapel Hill
Sonia Davis, DrPh. Phone: (919) 966-8333
Director, Collaborative Studies Coordinating Center
Department
of Biostatistics
University of North Carolina at Chapel Hill
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Request for OMB Approval of |
| Author | HEART5 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2025 OMB.report | Privacy Policy