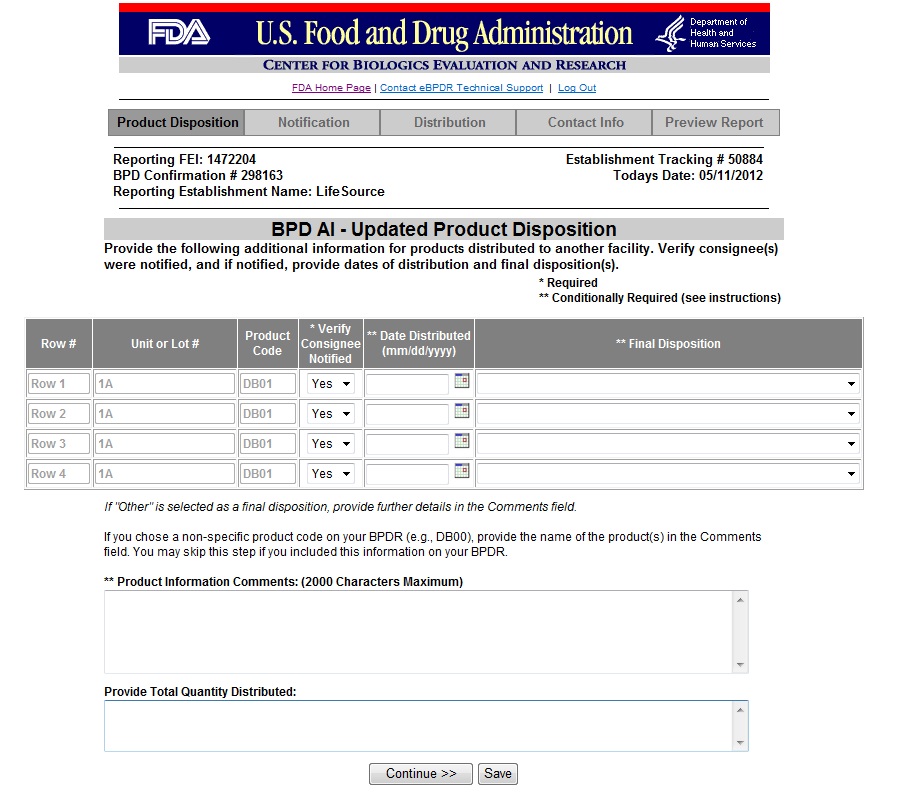
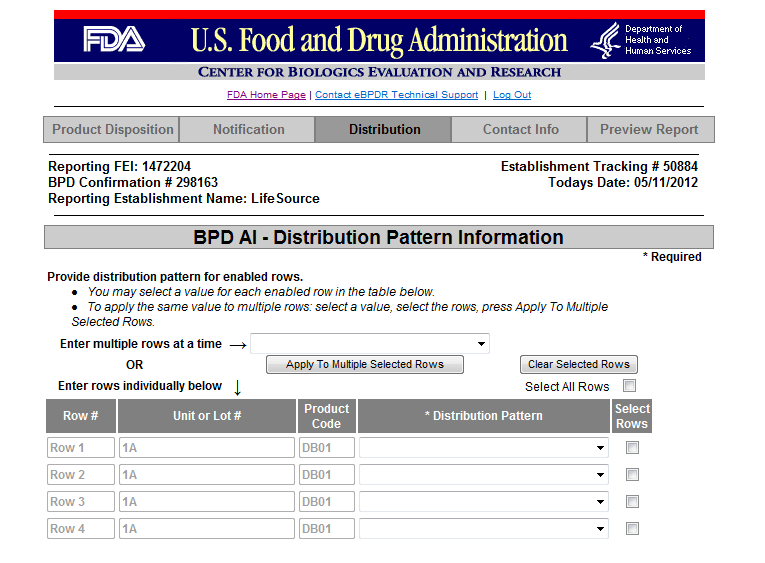
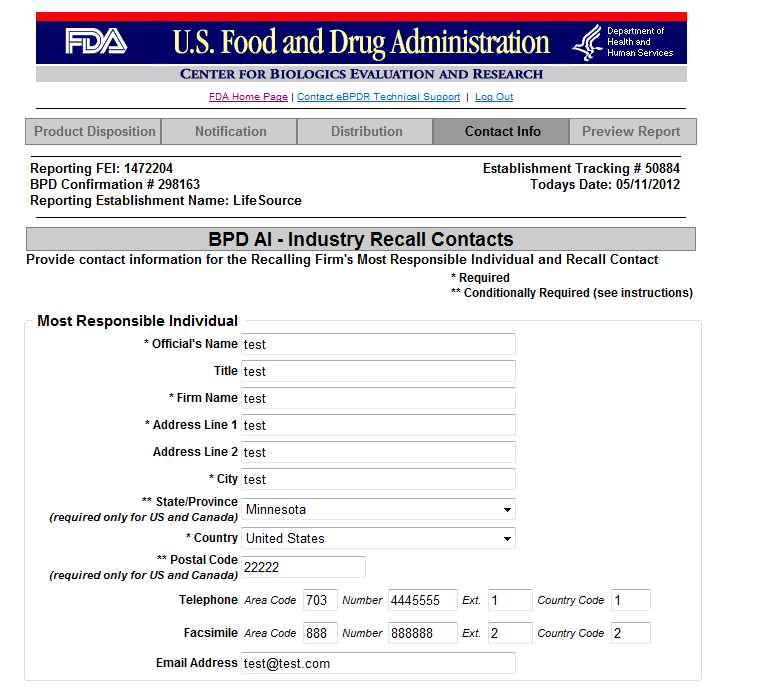
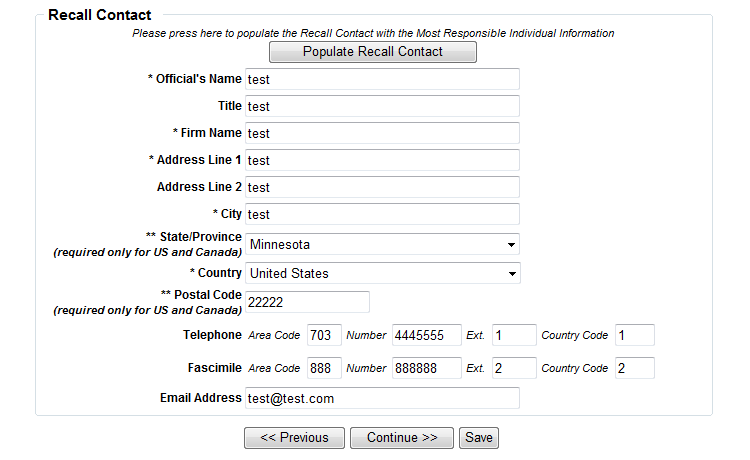
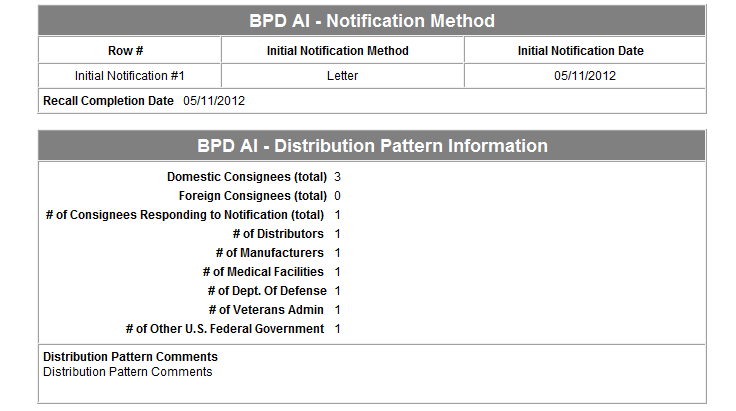
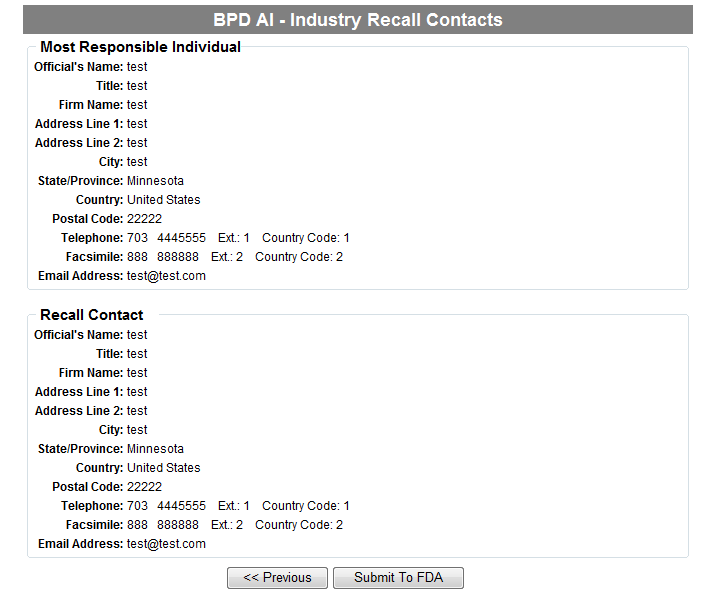
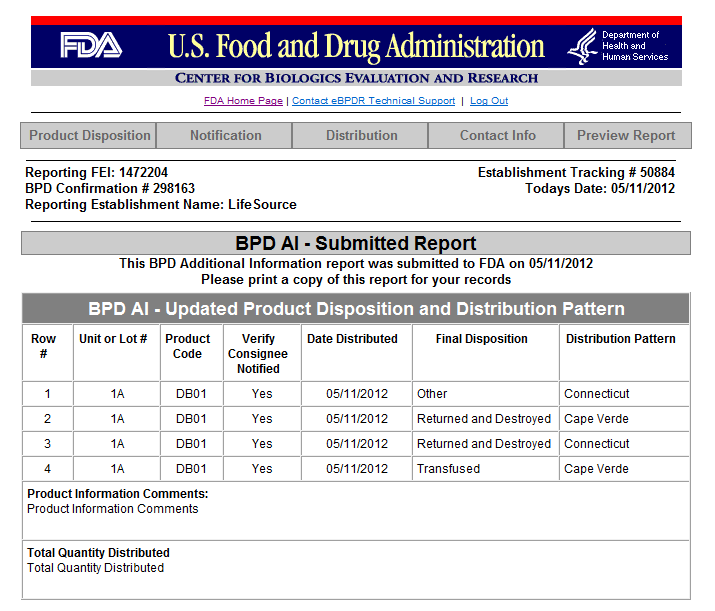
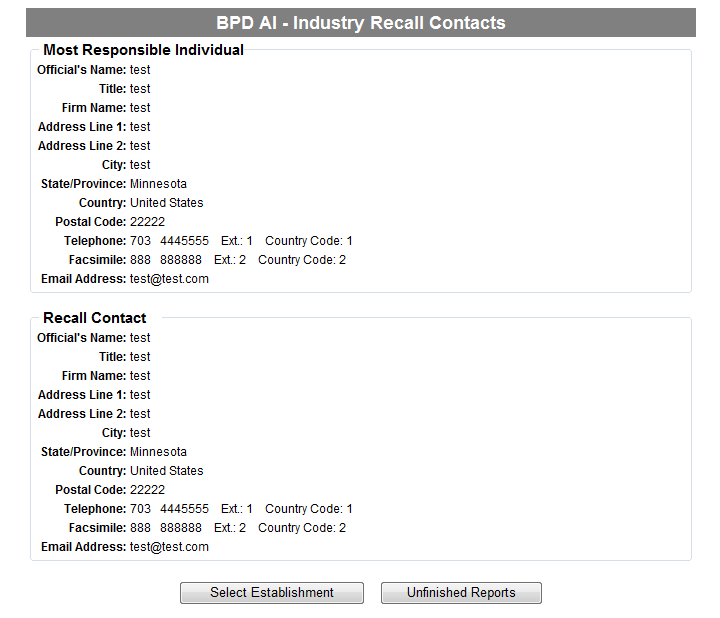
Form 3486A Biological Product Deviation Report - Addendum
Biological Products: Reporting of Biological Product Deviations and Human Cells, Tissues, and Cellular and Tissue-Based Product Deviations; Form FDA 3486 and Addendum 3486A
EBPDR_AI_Screen_Shots 12-16-13
Form FDA 3486A Addendum
OMB: 0910-0458
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0458 can be found here:
© 2026 OMB.report | Privacy Policy