Att 3c - Summary of Changes
Att 3C_Summary of Proposed Changes.docx
National Program of Cancer Registries Program Evaluation Instrument
Att 3c - Summary of Changes
OMB: 0920-0706
NPCR Program Evaluation Instrument (NPCR PEI)
Summary of Proposed Changes for 2013-2015
(numbers correspond to the question number in the survey instrument)
Based on the 2012 changes in Program Standards, it is expected that some of the questions below will be deleted or revised. In addition, questions where 100% of the states are now meeting the related standard will be deleted. Questions to be added will be based on the new FOA 1205 requirements, new NPCR Program Standard requirements and registry operational practices related primarily to informatics, such as incorporation of data from electronic medical records
Based on the 2012 changes in Program Standards, some questions will be deleted and others will be revised to be in line with the new standards. Several new questions have been proposed based on the new FOA 1205 requirements, new NPCR Program Standard requirements and registry operational practices related primarily to informatics, such as incorporation of data from electronic medical records.
Purpose Statement
Based on CDC’s Updated Guidelines for Evaluating Public Health Surveillance Systems, the PEI monitors the integration of surveillance, registry operations and health information systems, the utilization of established data standards, and the electronic exchange of health data. Data provided by this report can be used for public health action, program planning and evaluation, and research hypothesis formulation. (Highlighted word added)
2. Total Number of Staff (added)
3. Does your state/territory have a current law authorizing a population-based central cancer registry? (Program Standard I.a.)--- (Discontinue because 100% of the states are now meeting the related standard - will be deleted)
4. Does your state/territory have current legislation or regulations in support of all 8 criteria of the Public Law authorizing the NPCR? (Program Standard I.b.) --- (Discontinue because 100% of the states are now meeting the related standard - will be deleted)
5a. Does your state/territory’s current law/regulation include any penalties regarding reporting
compliance as mandated by current legislation or regulations?
5d. Have the law/regulations been revised in the past two years?
6a. With passage of Public Law 107-260 (the Benign Brain Tumor Cancer Registry Amendment Act), NPCR-funded registries are required to collect data on benign brain tumors beginning in diagnosis year 2004. Do regulations or legislation in your state or territory authorize you to collect data on benign brain tumors?
10. Does your CCR maintain an operational manual that describes registry operations, policies and
procedures that, at a minimum, contains the following? (Program Standard II.a.) Check all that apply:--
(Program Standards are being revised and this section will be revised to match)
11. Delete
12. Delete
13. Delete
Additional questions asked in table:
16. Table has been revised
Physician Groups (Centers/Clinics/Practices) – Use this top section to report specialty physicians counted using the Practice Method*** |
|||
a. Physician Specialty |
b. Number reporting* at the end of 20XX |
c. Number currently reporting* |
d. Number reporting electronically** |
Health Centers (HIS, Tribal) |
|
|
|
Surgery |
|
|
|
Independent Radiation Therapy |
|
|
|
Hematology |
|
|
|
Medical Oncology |
|
|
|
Urology |
|
|
|
Dermatology |
|
|
|
Gastroenterology |
|
|
|
Other |
|
|
|
Individual Physicians – Use this lower section to report specialty physicians counted using the Individual Physician Method*** |
|||
a. Physician Specialty |
b. Number reporting at the beginning for 20XX |
c. Number currently reporting |
d. Number reporting electronically |
Radiation Oncologists |
|
|
|
Medical Oncologists |
|
|
|
Urologists |
|
|
|
Dermatologists |
|
|
|
Gastroenterologists |
|
|
|
Other |
|
|
|
|
|
|
|
17. Delete
18. Delete
21. Delete
23. Delete
25a. New Question: How many providers have contacted you regarding meaningful use?
Of those who have contacted you, how many have signed on/ have initiated the Meaningful Use process with your registry?
26. Does your CCR use and require the following standardized, CDC-recommended data formats for the electronic exchange of cancer data from reporting sources. (Program Standards IV.a.):
a. Hospital Reports (The NAACCR record layout version specified in Standards for Cancer Registries Volume II: Data Standards and Data Dictionary)?
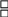 Yes
Yes
No
b. Pathology reports (NAACCR Standards for Cancer Registries Volume V: Pathology Laboratory Electronic
Reporting)?
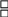 Yes
Yes
 No
No
Not Applicable, not receiving electronic pathology reports
c. Ambulatory healthcare providers using electronic health records (Implementation Guide for
Ambulatory Healthcare Provider Reporting to Central Cancer Registries)
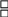 Yes
Yes
 No
No
Not Applicable, not receiving Ambulatory healthcare providers
27. Do your interstate data exchange procedures meet the following minimum criteria?
(Program Standards V.d.): (revised question)
Insert: What type of secure encrypted Internet-based system is used? (Drop down list: PHINMS, secure FTP, WebPlus, HTTPS, N-IDEAS, secure encrypted e-mail, other)
34b. Did your CCR match by tumor (site/histology) and not just by patient identifying information? (Combine 34a and 34b)
37c. (New Questions) Does your CCR have an established threshold for percent of records passing edits on incoming submissions?
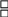 Yes
Yes
No
37d. If “Yes” what is the threshold?
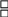 100%
100%
90%
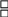 80%
or greater
80%
or greater
Less than 80%
39a. Within 24 months of the end of the diagnosis year with data that are 95% complete, did your CCR calculate incidence rates and counts (revise to be consistent with question 38) in an electronic data file or report? (The report should include, at a minimum, age-adjusted incidence rates and age-adjusted mortality rates for the diagnosis year by sex for SEER site groups, and, where applicable, by sex, race, and ethnicity). (Program Standard VII.b.) (Revised question)
39b. Within 24 months of the end of the diagnosis year with data that are 95% complete, did the CCR create biennial reports providing data on stage and incidence by geographic area with an emphasis on screening-amenable cancers and cancers associated with modifiable risk factors (e.g., tobacco, obesity, HPV). (New Question)
Yes (both years)
No (both years)
One year
40. Has the CCR, state health department, or its designee used registry data for planning and evaluation of cancer control objectives in at least three of the following ways in the past year: (Program Standard VIII.c.) Choices below have been revised
Comprehensive cancer control detailed incidence/mortality estimates,
Detailed incidence/mortality by stage and geographic area
Collaboration with cancer screening programs for breast, colorectal, or cervical cancer,
Health event investigation(s),
Needs assessment/program planning (e.g., Community Cancer Profiles),
Program evaluation,
Epidemiologic studies
40b. If “yes,” indicate the number of times data was used for each category in the table below: (Choices below have been revised)
Data Use Category |
Number per Year |
Comprehensive cancer control |
|
Detailed incidence/mortality estimates |
|
Linkage with a statewide cancer screening program |
|
Health event investigation(s) |
|
Needs assessment/program planning |
|
Program evaluation |
|
Epidemiologic studies |
|
Other, describe: |
|
47a. (New Question) With which chronic disease programs does your CCR collaborate? So we can capture other collaborations)
 .
Tobacco
Control
.
Tobacco
Control
 .
Oral
Health
.
Oral
Health
. Diabetes
. Physical Activity and Nutrition/Obesity
Radiation Control
. Environmental Health
. Infectious disease (HIV AIDS, HPV, hepatitis
Other
48. Delete
52. Delete Question
53. Does your CCR conduct at least one of the following advanced activities: Check all that apply:
(options have been revised)
 .
Survival
analysis
.
Survival
analysis
. Quality of care studies
. Clinical Studies
. Publication of research studies using registry data
. Geo-coding to latitude and longitude to enable mapping
. Other healthcare data reporting. Describe

 .
Other
innovative
uses
of
registry
data
such
as.
Survivorship Care Plan.
Describe
.
Other
innovative
uses
of
registry
data
such
as.
Survivorship Care Plan.
Describe

 .
None
of
the
above
.
None
of
the
above
58. With which databases did your CCR link its records in 2012 for follow-up or some other purpose? Check all that apply (options were revised)
State Vital Statistics
National Death Index
Department of Motor Vehicles
Department of Voter Registration
Indian Health Service
Medicare (Health Care Financing Administration)
Medicare Physician Identification and Eligibility Registry
Medicaid
CDC’s National Breast and Cervical Cancer and Early Detection Program
Insurance Claim Databases (IE: BC&BS, Kaiser, Managed Care Organization, fee for service etc)
Hospital Discharge Database
Other, specify: _________________________________
Hospital Radiation Therapy Dept
Hospital Disease Indices
None
57. With which databases did your CCR link its records in 2012 for follow-up or some other purpose?
(options have been revised)
 .
State
Vital
Statistics
.
State
Vital
Statistics
 .
National
Death
Index
.
National
Death
Index
. Department of Motor Vehicles
. Department of Voter Registration
. Indian Health Service
 .
Medicare
(Health
Care
Financing
Administration)
.
Medicare
(Health
Care
Financing
Administration)
Medicare Physician Identification and Eligibility Medicaid
 .
CDC’s
National Breast
and
Cervical
Cancer
and Early Detection Program
.
CDC’s
National Breast
and
Cervical
Cancer
and Early Detection Program
Insurance Claim Databases (IE: BC&BS, Kaiser,Managed Care Organization, fee for service Etc) Hospital Discharge Database Other, specify: None
58. In a given calendar year, what percentage of your total pathology reports (both electronic and paper) received was sent by the following independent laboratories? (Estimates acceptable if exact % not available) (options have been revised)
-
Laboratory Corporation of America (LabCorp)/US Labs, Dianon,
%
Quest Diagnostics:
%
Bostwick Laboratories:
%
Mayo Laboratories:
%
- Bioreference
%
GI Pathology
%
AmeriPath
%
Clarent
%
Miraca Labs
%
CBL Path
%
Other
%
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy