12 MO Child Saliva Collection Instructions (12 MO)
12 MO Child Saliva Instructions 20120413.docx
Provider-Based Sampling Feasibility Study for the Vanguard (Pilot) Study and Data Collection Updates for the National Children's Study (NICHD)
12 MO Child Saliva Collection Instructions (12 MO)
OMB: 0925-0593
OMB #: 0925-0593
OMB Expiration Date: 07/31/ 2013
Biospecimen Child Saliva Collection Instructions

The National Children’s Study
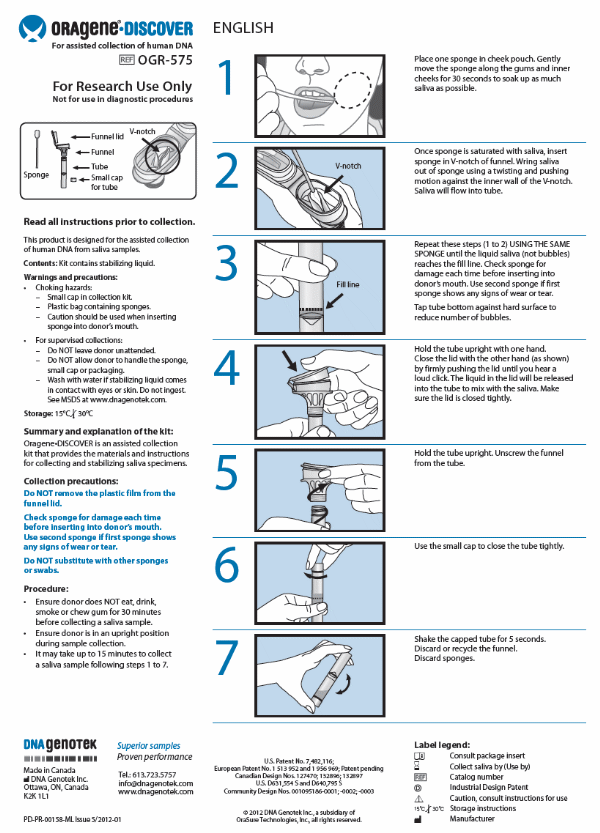
Child Saliva Collection Instructions
-
Instructions:

Insert local contact information label here.
Public reporting burden for this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0593). Do not return the completed form to this address.
BIO
Child Saliva Collection Instructions (EHPBHI), MDES 3.0 Spring 2012,
V1.0
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-30 |
© 2025 OMB.report | Privacy Policy