Revised FOA with Track Changes
0990-0342 FOA_State Health Information Exchange Cooperative Agreement Program_Sept3proposedchanges (2).doc
Section 3013 State Health Information Exchange Cooperative Agreement Program: Full Application
Revised FOA with Track Changes
OMB: 0990-0342
American Recovery and Reinvestment Act of 2009, Title XIII - Health Information Technology, Subtitle B—Incentives for the Use of Health Information Technology, Section 3013, State Grants to Promote Health Information Technology
State Health Information Exchange Cooperative Agreement Program
Funding Opportunity Announcement
Office of the National Coordinator for Health Information Technology
Department of Health and Human Services
2009
American Recovery and Reinvestment Act of 2009:
State Health Information Exchange Cooperative Agreement Program
Table of Contents
I. Funding Opportunity Description 6
D. Program Structure and Approach 9
b) Five Domains Supporting the Program 10
2. Specific Requirements for the First Two Years 11
3. State Plans – Strategic & Operational Plan 13
b) Ongoing Planning Requirements 15
E. State Plan Preparation Activities for Application Submission 15
1. Self - Assessment of the State’s Current Status 15
2. Application Submission, Review, and Funding Process 18
F. Key Considerations & Challenges for HIE Implementations 19
1. Medicaid and Medicare Coordination 19
III.Eligibility Information 23
1. Example Match Computation 24
C. Responsiveness and Screening Criteria 25
1. Application Responsiveness Criteria 25
2. Application Screening Criteria 25
IV.Application and Submission Information 25
B. Address to Request Application Package 25
C. Content and Form of Application Submission 26
3. Tips for Writing a Strong Application 27
b) Proposed Project Summary 29
c) Required Performance Measures and Reporting 30
f) Organizational Capability Statement 32
7. Collaborations and Letters of Commitment from Key Participating Organizations and Agencies 33
8. Budget Narrative/Justification 33
D. Submission Dates and Times 33
E. Intergovernmental Review 34
G. Other Funding Information 34
3. Performance-Based Funding 35
H. Other Submission Requirements 36
I. Summary of Required Attachments 36
V. Application Review Information 36
B. Review and Selection Process 38
VI.Award Administration Information 38
B. Administrative and National Policy Requirements 39
1. HHS Grants Policy Statement 39
2. Financial Status Reports 39
D. Cooperative Agreement Terms and Conditions of Award 41
1. Cooperative Agreement Roles and Responsibilities 41
E. American Recovery and Reinvestment Act of 2009 43
1. HHS Standard Terms and Conditions 43
2. Preference for Quick Start Activities 44
5. Civil Rights Obligations 44
6. Disclosure of Fraud or Misconduct 44
7. Responsibilities for Informing Sub-recipients 44
B. Detailed Guidance for Strategic and Operational Plans 51
1. Detailed Guidance for the Strategic Plan 51
2. Detailed Guidance for the Operational Plan 53
a) General Topic Requirements 54
C. Required Content for Letter of Intent to Apply 56
E. Suggested Format for Letter of Support from Critical Stakeholders 59
F. Privacy and Security Resources 60
G. ARRA-Required Performance Measures 62
H. Public and Private Sector Models for Governance and Accountability 63
J. Budget Narrative/Justification, Page 1 – Sample Format with EXAMPLES 71
K. Budget Narrative/Justification ––Template 74
L. Instructions for Completing the Project Summary/Abstract 75
M. Survey instructions on Ensuring Equal Opportunity for Applicants 76
Opportunity Overview
Department of Health and Human Services (HHS
Office of the National Coordinator for Health Information Technology (ONC)
Office of Programs and Coordination
Funding Opportunity Title: American Recovery and Reinvestment Act of 2009, State Grants to
Promote Health Information Technology Planning and Implementation Projects
Announcement Type: Initial
Funding Opportunity Number: EP-HIT-09-001
Catalog of Federal Domestic Assistance (CFDA) Number: 93.719
-
Item to Submit
Date1
Section Reference
Letter of Intent
September 11, 2009, by 5:00pm EST
Section IV.B.1 – Application and Submission Information
Application
October 16, 2009 by 5:00pm EST
Section IV – Application and Submission Information
Award Announcements
December 15, 2009
IV.A – Award Administration Information
Anticipated Project Start Date
Beginning January 15, 2010
IV.A – Award Administration Information
Executive Summary
The State Cooperative Agreements to Promote Health Information Technology: Planning and Implementation Projects are to advance appropriate and secure health information exchange (HIE) across the health care system. Awards will be made in the form of cooperative agreements to states or qualified State Designated Entities (SDEs). The purpose of this program is to continuously improve and expand HIE services over time to reach all health care providers in an effort to improve the quality and efficiency of health care. Cooperative agreement recipients will evolve and advance the necessary governance, policies, technical services, business operations and financing mechanisms for HIE over a four year performance period. This program will build off of existing efforts to advance regional and state level HIE while moving towards nationwide interoperability.
Total funding for this initiative is $564,000,000. States (including territories) or their non-profit SDEs may apply, as designated by the state. No more than one award will be made per state. States may choose in enter into multi-state arrangements.
I.Funding Opportunity Description
A.Background
On February 17, 2009, the President signed the American Recovery and Reinvestment Act of 2009 (ARRA). This statute includes The Health Information Technology for Economic and Clinical Health Act of 2009 (the HITECH Act) that sets forth a plan for advancing the appropriate use of health information technology to improve quality of care and establish a foundation for health care reform. The Office of the National Coordinator for Health Information Technology (ONC) was statutorily created by the HITECH Act within the U.S. Department of Health and Human Services (HHS). ONC serves as the principal federal entity charged with coordinating the overall effort to implement a nationwide health information technology infrastructure that allows for the electronic use and exchange of health information.
The HITECH Act authorizes the Centers for Medicare & Medicaid Services (CMS) to administer incentives to eligible professionals (EPs) and hospitals for meaningful use of electronic health records (EHRs).2 These incentives are anticipated to drive adoption of EHRs needed to reach the goal of all Americans having secure EHRs. To achieve the vision of a transformed health system that health information technology (HIT) can facilitate, there are three critical short-term prerequisites:
Clinicians and hospitals must acquire and implement certified EHRs in a way that fully integrates these tools into the care delivery process;
Technical, legal, and financial supports are needed to enable information to flow securely to wherever it is needed to support health care and population health; and,
A skilled workforce needs to support the adoption of EHRs, information exchange across health care providers and public health authorities, and the redesign of work-flows within health care settings to gain the quality and efficiency benefits of EHRs, while maintaining individual privacy and security.
Priority Programs. The HITECH Act also authorizes the establishment of several new grant programs that will provide resources to address these prerequisites. Together, they are intended to facilitate the adoption and use of EHRs by providing technical assistance, the capacity to exchange health information, and the availability of trained professionals to support these activities. These priority grant programs are:
Health Information Technology Extension Program (Extension Program), authorized by Section 3012 of the Public Health Service Act (PHSA) as amended by ARRA - will establish a collaborative consortium of Health Information Technology Regional Extension Centers (Regional Centers) facilitated by the national Health Information Technology Research Center (HITRC). The Extension Program will offer providers across the nation technical assistance in the selection, acquisition, implementation, and meaningful use of an EHR to improve health care quality and outcomes.
State Grants to Promote Health Information Technology (State Health Information Exchange Cooperative Agreements Program), authorized by Section 3013 of the PHSA as amended by ARRA - to promote health information exchange (HIE) that will advance mechanisms for information sharing across the health care system. This is the topic of this Funding Opportunity Announcement. Complete statutory language for this section is available in Appendix A of this document.
Information Technology Professionals in Health Care (Workforce Program), authorized by Section 3016 of the PHSA as amended by ARRA - to fund the training and development of a workforce that will meet short-term HITECH Act programmatic needs.
Meaningful Use Incentives and Related Criteria. The priority grant programs are fundamental to realizing the promise of meaningful use of HIT that leads to improved quality, efficiency and safety of health care. Under the HITECH Act, an eligible professional or hospital is considered a "meaningful EHR user" if they use certified EHR technology in a manner consistent with criteria established by the Secretary, including but not limited to e-prescribing through an EHR, and the electronic exchange of information for the purposes of quality improvement, such as care coordination. In addition, eligible professionals and hospitals must submit clinical quality and other measures to HHS.
Meaningful use incentives will be available to healthcare providers beginning in FY 2011 based on their Medicare and Medicaid coverage status and other statutorily defined factors. This includes eligible health care professionals and acute care hospitals and takes into consideration adjustment factors for children’s hospitals and critical access hospitals. The detailed criteria to qualify for meaningful use incentive payments will be established by the Secretary of HHS through the formal notice-and-comment rulemaking process.
The HITECH Act also requires these meaningful use criteria to become more stringent over time. In 2015, providers are expected to have adopted and be actively utilizing an EHR in compliance with “meaningful use”or they will be subject to financial penalties under Medicare. The information exchange requirements for the meaningful use EHR incentives, as specified in the regulation currently under devleopment, will inform a strategic framework for this program. Any goals, objectives and corresponding measures of meaningful use that require HIE over time will be the reference point for states and/or SDEs as they develop and update their plans to build capacity for HIE for all providers across their states.
The implementation of the HITECH Act provides requirements for meaningful use of EHRs that will guide both state and federal efforts to advance HIE in ways that enable eligible health care providers to qualify for Medicare and Medicaid incentives and improve the quality and efficiency of health care.
B.Purpose
Widespread adoption and meaningful use of HIT is one of the foundational steps in improving the quality and efficiency of health care. The appropriate and secure electronic exchange and consequent use of health information to improve quality and coordination of care is a critical enabler of a high performance health care system. The overall purpose of this program, as authorized by Section 3013 of the PHSA, as added by ARRA, is to facilitate and expand the secure, electronic movement and use of health information among organizations according to nationally recognized standards. The governance, policy and technical infrastructure supported through this program will enable standards-based HIE and a high performance health care system.
This program will be a federal-state collaboration aimed at the long-term goal of nationwide HIE and interoperability. To this end, ONC intends to award cooperative agreements to states or SDEs to meet local health care provider, community, state, public health and nationwide information needs. Each state’s cooperative agreement award will be for both planning and implementation, except for states that have a plan approved by the National Coordinator prior to award in which case they would only receive implementation funding.. ONC will award no more than one cooperative agreement per state; however groups of states may combine their efforts into one application. The cooperative agreement approach allows for a greater level of coordination and partnership between ONC and states or their SDEs. Please note: For purposes of this program agreement, “state” includes the District of Columbia and the U.S. territories – Puerto Rico, U.S. Virgin Islands, Guam, the Northern Mariana Islands, and American Samoa.
The cooperative agreements will focus on developing the statewide policy, governance, technical infrastructure and business practices needed to support the delivery of HIE services. The resulting capabilities for healthcare-providing entities to exchange health information must meet the to-be-developed Medicaid and Medicare meaningful use requirements for health care providers to achieve financial incentives.
C.The Roles of State Government, Federal Government, and the Private Sector in Advancing Health Information Exchange
State government, federal government and the private sector will all play important roles in advancing HIE among health care providers, public health and those providing patient engagement services (such as Personal Health Records) in a state enabled by this grant program. Many states have already made significant progress in developing governance, policies, and technical capacity for HIE among health care providers. Moving forward, states will continue to play a critical leadership role by determining a path and a model for exchange of health information that leverages existing regional and state efforts and is based on HHS-adopted standards and certification criteria. States will develop and implement Strategic and Operational Plans that will ensure that a comprehensive set of actions will result in adoption of HIE to enable providers to meet the HIE meaningful use criteria to be established by the Secretary through the rulemaking process (for up-to-date publicly available information on meaningful use, see: http://healthit.hhs.gov/meaningfuluse).
States will also be expected to use their authority, programs, and resources to:
Develop state level directories and enable technical services for HIE within and across states.
Remove barriers and create enablers for HIE, particularly those related to interoperability across laboratories, hospitals, clinician offices, health plans and other health information trading partners.3
Convene health care stakeholders to ensure trust in and support for a statewide approach to HIE.
Ensure that an effective model for HIE governance and accountability is in place.
Coordinate an integrated approach with Medicaid and state public health programs to enable information exchange and support monitoring of provider participation in HIE as required for Medicaid meaningful use incentives.
Develop or update privacy and security requirements for HIE within and across state borders.
States will have the option to designate a non-profit entity to assume most of these responsibilities, however; state government at a minimum is expected to coordinate activities across Medicaid and state public health programs, so as to not duplicate efforts and to ensure integration and support of a unified approach to information exchange.
The federal government will continue to advance interoperability and health information exchange through a variety of regulatory and programmatic activities. HHS will:
Collaborate with states and SDEs to promote, monitor and share efficient, scalable and sustainable mechanisms for HIE within and across states.
Conduct a national program evaluation and offer technical assistance for state-level evaluations in an effort to implement lessons learned that will ensure appropriate and secure HIE resulting in improvements in quality and efficiency.
Harmonize and regulate standards and certification criteria to enable interoperability and HIE.
Provide technical assistance to states and SDEs.
Coordinate efforts across states and regions in effort to support nationwide HIE.
Advance standards-based HIE through the development of the Nationwide Health Information Network (NHIN).4
Establish a governance mechanism for the NHIN informed by HIE activities across states, and regions, including entities participating in the NHIN.
The private sector will participate in state level strategic planning and develop innovative solutions to HIE among health care providers. States will need to specify the role of various health care stakeholders in their Strategic and Operational plans and hold stakeholders accountable for their contributions to the development and universal adoption of HIE. For example, a state could rely on HIT vendors to develop and operate state level network services for HIE, health plans to provide incentives to clinicians and hospitals for HIE, and Regional Centers to provide technical assistance to health care providers to help them implement the workflow and technical changes to the providers’ processes needed to successfully connect to the available HIE infrastructure.
Medicare and Medicaid meaningful use incentives are anticipated to create demand for products and services that enable HIE among eligible providers. States can use convening, regulatory, procurement, and other policy levers to also incentivize information exchange for the “trading partners” (e.g., laboratories, pharmacies, radiology) of eligible providers. The resulting demand for health information exchange will likely be met by an increased supply of marketed products and services to enable HIE, resulting in a competitive marketplace for HIE services. It is also important for the private sector to develop innovative products and approaches for HIE that meet the provider demands and needs over time, while enabling the measurement and improvements in health care quality and efficiency.
D.Program Structure and Approach
1.Summary of Program
This program is focused on preparing states to support their providers in achieving goals, objectives, and measures related to HIE. Information exchange is both a statutory requirement for meaningful use incentives and critical to enabling care coordination and other improvements to quality and efficiency. States participating in the State HIE Program will begin at different stages of maturity working towards interoperable HIE. Some will be fully operational, while others will just be starting to build the necessary capacity.
ONC will award up to one cooperative agreement per state to cover both planning and implementation of statewide health information exchange. However, groups of states may combine their efforts into one application.
The process of building HIE capacity begins with states assessing their current state of readiness. Once a state determines from where it is starting, it can begin to map out a critical path to developing HIE for all health care providers throughout the state.
The work associated with enabling statewide HIE services is complicated and may become overwhelming if not broken down into manageable components. An "all at once" approach is not recommended, but instead this program will allow for an incremental approach to ensure continuous improvement and expansion of HIE capabilities. To further enable an incremental approach, the work necessary for realizing HIE falls into five domains. These domains of HIE include: governance, finance, technical infrastructure, business and technical operations, and legal/policy (these are further described below in Section I.D.1.b).
a)The Pathway to HIE
The HITECH Act specifies that information exchange is required for meaningful use and that meaningful use measures become more stringent over time.
Based on these statutory requirements ONC recommends that a pathway for realizing statewide HIE be considered in a series of stages, consistent with the statutory requirements for meaningful use. Specific requirements and associated criteria for meaningful use will be proposed and advanced through a CMS rule-making process during Fiscal Year 2010.
Based on the rulemaking process, future program guidance will specify program requirements to achieve the statutory requirements set forth in the HITECH Act, which include e-prescribing, care coordination, quality reporting, and other HIE services that improve quality and efficiency.
b)Five Domains Supporting the Program
Developing capacity for HIE is an incremental process that requires demonstrated progress across five essential domains: governance, finance, technical infrastructure, business and technical operations, and legal/policy. To realize HIE, states will need to plan, implement and evaluate activities across all five HIE domains. The goals, strategies and objectives of HIE will guide the implementation and evaluation activities. The extent to which states have to “implement” these activities will vary with their approach to HIE. In some cases, they will be overseeing and evaluating the development and implementation of network services undertaken by the private sector.
Description of the Five Domains:
Governance – This domain addresses the functions of convening health care stakeholders to create trust and consensus on an approach for statewide HIE and to provide oversight and accountability of HIE to protect the public interest. One of the primary purposes of a governance entity is to develop and maintain a multi-stakeholder process to ensure HIE among providers is in compliance with applicable policies and laws.
Finance - This domain encompasses the identification and management of financial resources necessary to fund health information exchange. This domain includes public and private financing for building HIE capacity and sustainability. This also includes but is not limited to pricing strategies, market research, public and private financing strategies, financial reporting, business planning, audits, and controls.
Technical Infrastructure – This domain includes the architecture, hardware, software, applications, network configurations and other technological aspects that physically enable the technical services for HIE in a secure and appropriate manner.
Business and Technical Operations – The activities in this domain include but are not limited to procurement, identifying requirements, process design, functionality development, project management, help desk, systems maintenance, change control, program evaluation, and reporting. Some of these activities and processes are the responsibility of the entity or entities that are implementing the technical services needed for health information exchange; there may be different models for distributing operational responsibilities.
Legal/Policy – The mechanisms and structures in this domain address legal and policy barriers and enablers related to the electronic use and exchange of health information. These mechanisms and structures include but are not limited to: policy frameworks, privacy and security requirements for system development and use, data sharing agreements, laws, regulations, and multi-state policy harmonization activities. The primary purpose of the legal/policy domain is to create a common set of rules to enable inter-organizational and eventually interstate health information exchange while protecting consumer interests.
c)Continuous Improvement
Section 3013(h) of the HITECH Act, requires the Secretary to complete an annual evaluation of the activities conducted under this program and, in awarding cooperative agreements under section 3013, implement lessons learned from the evaluations. This will shape future program guidance and enable continuous improvements to the program. Additionally, ONC will collaborate with the states and provide technical assistance in order to ensure that lessons learned are implemented in a way that promotes quality and efficiency improvement through secure and appropriate electronic exchange of health information.
2.Specific Requirements for the First Two Years
The first two years of this program are critical for HIE capacity building. As such, it is expected that states and SDEs will make considerable progress in achieving a critical mass of providers participating in HIE. To this end, a majority of the funding will be available for drawdown in the first two years, based on milestones and specific measures achieved in this period.
The milestones and measures will be based in part on the progress made across the five domains of HIE. In the first two years, states or SDEs will be responsible for developing and implementing plans that take into account the necessary progress to be made in all five domains to assure HIE is sufficient to meet HIE meaningful use criteria to be established by the Secretary through the rulemaking process. It is anticipated that states or SDEs will build off of regional health information organizations where they exist and other HIE mechanisms that will ultimately enable full interoperability and exchange across the state.
While a state or an SDE may or may not be the entity to implement and operate technical services to support HIE, they are required to act as the governance entity responsible for ensuring that HIE capacity will be developed with appropriate oversight and accountability. Thus, the state or SDE must develop and implement a plan that provides reasonable assurance that the HIE requirements for meaningful use will be attained by 2015, when Medicare penalties begin for providers that have not achieved meaningful use of EHRs.
States’ and SDEs’ responsibilities include establishing multi-stakeholder support for a pathway toward statewide HIE among healthcare providers and determining the role of the private sector in providing and maintaining the services. To the extent that the private sector is responsible for developing and implementing HIE services, the state or SDE must ensure that the responsible private organizations do so in a manner that is compliant with relevant HHS adopted standards and all applicable policies for interoperability, privacy and security. Additionally, the state or SDE must ensure the private sector efforts to advance HIE are efficient and scalable such that they will cover the providers in the state by 2015.
Key accomplishments to be met by the recipients in the first two years include:
Governance
Establish a governance structure that achieves broad-based stakeholder collaboration with transparency, buy-in and trust.
Set goals, objectives and performance measures for the exchange of health information that reflect consensus among the health care stakeholder groups and that accomplish statewide coverage of all providers for HIE requirements related to meaningful use criteria to be established by the Secretary through the rulemaking process. .
Ensure the coordination, integration, and alignment of efforts with Medicaid and public health programs through efforts of the State Health IT Coordinators.
Establish mechanisms to provide oversight and accountability of HIE to protect the public interest.
Account for the flexibility needed to align with emerging nationwide HIE governance that will be specified in future program guidance.
Finance
Develop the capability to effectively manage funding necessary to implement the state Strategic Plan. This capability should include establishing financial policies and implementing procedures to monitor spending and provide appropriate financial controls.
Develop a path to sustainability including a business plan with feasible public/private financing mechanisms for ongoing information exchange among health care providers and with those offering services for patient engagement and information access.
Technical Infrastructure
Develop or facilitate the creation of a statewide technical infrastructure that supports statewide HIE. While states may prioritize among these HIE services according to its needs, HIE services to be developed include:
Electronic eligibility and claims transactions
Electronic prescribing and refill requests
Electronic clinical laboratory ordering and results delivery
Electronic public health reporting (i.e., immunizations, notifiable laboratory results)
Quality reporting
Prescription fill status and/or medication fill history
Clinical summary exchange for care coordination and patient engagement
Leverage existing regional and state level efforts and resources that can advance HIE, such as master patient indexes, health information organizations (HIOs), and the Medicaid Management Information System (MMIS).
Develop or facilitate the creation and use of shared directories and technical services, as applicable for the state’s approach for statewide HIE. Directories may include but are not limited to: Providers (e.g., with practice location(s), specialties, health plan participation, disciplinary actions, etc), Laboratory Service Providers, Radiology Service Providers, Health Plans (e.g., with contact and claim submission information, required laboratory or diagnostic imaging service providers, etc.). Shared Services may include but are not limited to: Patient Matching, Provider Authentication, Consent Management, Secure Routing, Advance Directives and Messaging.
Business and Technical Operations
Provide technical assistance as needed to HIOs and others developing HIE capacity within the state.
Coordinate and align efforts to meet Medicaid and public health requirements for HIE and evolving meaningful use criteria.
Monitor and plan for remediation of the actual performance of HIE throughout the state.
Document how the HIE efforts within the state are enabling meaningful use.
Legal/Policy
Identify and harmonize the federal and state legal and policy requirements that enable appropriate health information exchange services that will be developed in the first two years.
Establish a statewide policy framework that allows incremental development of HIE policies over time, enables appropriate, inter-organizational health information exchange, and meets other important state policy requirements such as those related to public health and vulnerable populations.
Implement enforcement mechanisms that ensure those implementing and maintaining health information exchange services have appropriate safeguards in place and adhere to legal and policy requirements that protect health information, thus engendering trust among HIE participants.
Minimize obstacles in data sharing agreements, through, for example, developing accommodations to share risk and liability of HIE operations fairly among all trading partners.
Ensure policies and legal agreements needed to guide technical services prioritized by the state or SDE are implemented and evaluated as a part of annual program evaluation.
While recipients will be required to report on specific reporting requirements and performance measurements, ONC will make particular note of progress at the end of the first two-year period. See Reporting Requirements and Performance Measures on pages 30 and 31 in this document.
3.State Plans – Strategic & Operational Plan
Section 3013 of the HITECH Act requires states or SDEs to submit, and receive approval of a “State Plan” in order to qualify for implementation funding. To carry out the intent of the Act, the State Plan is defined as consisting of two deliverables: A Strategic Plan and an Operational Plan. Both the Strategic and the Operational Plans must be approved by the National Coordinator for Health Information Technology.
Currently, there are various approaches across the country to advance standards-based HIE among health care providers, public health and those offering services for patient engagement and information access, as well as varying degrees of planning and implementation across states and regions. It is anticipated; therefore, that states’ plans will reflect the existing variety of HIE approaches and levels of readiness. Part of the application award process entails an assessment of the Strategic and Operational Plans to enable the federal government to enter into an appropriately tailored cooperative agreement with each state. To facilitate the consistent development or updating of Strategic and Operational Plans for the purposes of this program, please refer to detailed guidance in Appendix B.
a)Plan Overview
The Strategic and Operational Plans shall describe activities the state or SDE will complete to enable or implement HIE services that will allow for eligible providers to achieve success. Both the Strategic and Operational Plans shall be submitted by each state. For states that turn in multi-state plans, each state will be expected to have its own Strategic and Operational plan that demonstrate how the joint plan will unfold within that state’s jurisdiction.
This section provides a brief overview of what needs to be included in the Strategic and Operational Plans. More details are provided in Appendix B.
Strategic Plan
Each state or SDE must have a Strategic Plan that addresses the vision, goals, objectives and strategies addressing statewide HIE development. Plans to support HIT adoption may also be included in the Strategic Plan Inclusion of Health IT adoption in the Strategic Plan is valuable and provides for a more comprehensive approach for planning how to achieve connectivity across the state. The Strategic Plan must also address continuous improvement in realizing effective and secure HIE across health care providers.5
The Strategic Plan must address all five of the domains:
Governance
Finance
Technical infrastructure
Business and technical operations
Legal/policy
A detailed description of the requirements for the Strategic Plan is provided in Appendix B.
Operational Plan
The Operational Plan must contain details on how the Strategic Plan will be executed to enable statewide HIE. The specific actions and roles of various stakeholders in the development and implementation of HIE services must be included. In addition, the Operational Plan must include descriptions of any implementation activities to date with an explanation of how these prior activities fit into the state’s future plans for HIE.
The Operational Plan must address all five of the domains:
Governance
Finance
Technical infrastructure
Business and technical operations
Legal/policy
A detailed description of requirements for the Operational Plan is provided in Appendix B.
Upon award of the cooperative agreement, funds may be available to recipients to develop, revise and improve their plans. There will be future technical assistance and guidance regarding implementation and evaluation; however, the allocation of funds will be dependent on where states are in planning and implementation. This is further detailed in (Section I.D.1.a).
b)Ongoing Planning Requirements
In order to ensure project success, recipients should periodically review their Strategic and Operational Plans and make updates to the plans based on new requirements for HIE as determined through CMS rule making for meaningful use incentives. However, other events may also require revisions of state plans. For example, recipients should reassess plans when relevant state law is changed, when ONC releases new or revised program guidance, or when the project has deviated significantly from its original path. Reassessments and updated Strategic and Operational Plans shall be submitted annually. These reassessments should be done in collaboration with ONC to maximize understanding of state actions and ease of processing of state requests for modifications.
E.State Plan Preparation Activities for Application Submission
States with existing Strategic and Operational Plans should submit them as part of the application if they want to quickly move into implementation. State Strategic and Operational Plans will be a tool to monitor, communicate and track progress throughout the performance period. Though State Plans are not the only component of the application, they are critical.
1.Self - Assessment of the State’s Current Status
During the application process, applicants will evaluate the status of any existing Strategic and Operational Plans. For multi-state applications, states may submit comparable coordinated Strategic and Operational Plans. When states submit multi-state applications, their plans will be evaluated at both the multi-state and individual state level. The multi-state plan will be evaluated as a whole, but state plans must be sufficient at the individual state level as well.
Based on the state’s assessment of the status of its planning activities, each applicant must indicate in their application which of the following levels of planning most closely describes the state of their Strategic and Operational Plans. Based on the indicated levels of planning, states should proceed as described below.
Status of Planning Activity:
No existing Strategic Plan – Applicants must provide a detailed description of the activities needed to develop Strategic and Operational Plans as outlined in Appendix B and in future guidance. Recipients shall develop initial Strategic and Operational Plans and submit them within the first six to eight months of the project.
Existing Strategic Plan and/or Operational Plan that is not consistent with planning guidance – Applicants shall provide: 1) their current Strategic and/or Operational Plan, 2) a detailed description of the gaps in their current Strategic Plan and/or Operational Plan in comparison to the parameters outlined in Appendix B, and 3) an outline of the activities contemplated to revise the plans to be consistent with planning guidance. For applicants in this category that have already begun implementation activities, their current Operational Plan must also include an explanation of how they will proceed with concurrent planning and implementation activities. States shall submit an updated Strategic and Operational Plan addressing the deficiencies of their existing plans within three months of award.
Existing Strategic and/or Operational Plan that is consistent with planning guidance – Applicants shall submit their Strategic and/or Operational Plan for approval by the National Coordinator. For applicants that have already begun implementing a state HIT plan prior to receiving an award under this program, the Operational Plan shall also be submitted and must contain a description of the implementation activities to date and explain how they plan to proceed with continued implementation of the operational plan.
Sequence of Pre- and Post-Award Events throughout the Project:
The status of the state’s plans will determine what steps the state shall complete in submitting their application and any accompanying materials. This diagram below depicts the activities that will take place before (Pre-Award) and after (Post-Award) a cooperative agreement is signed. This process and the use of funding will vary depending on the current status of a state’s plan at the time that the application and supporting plans are submitted.
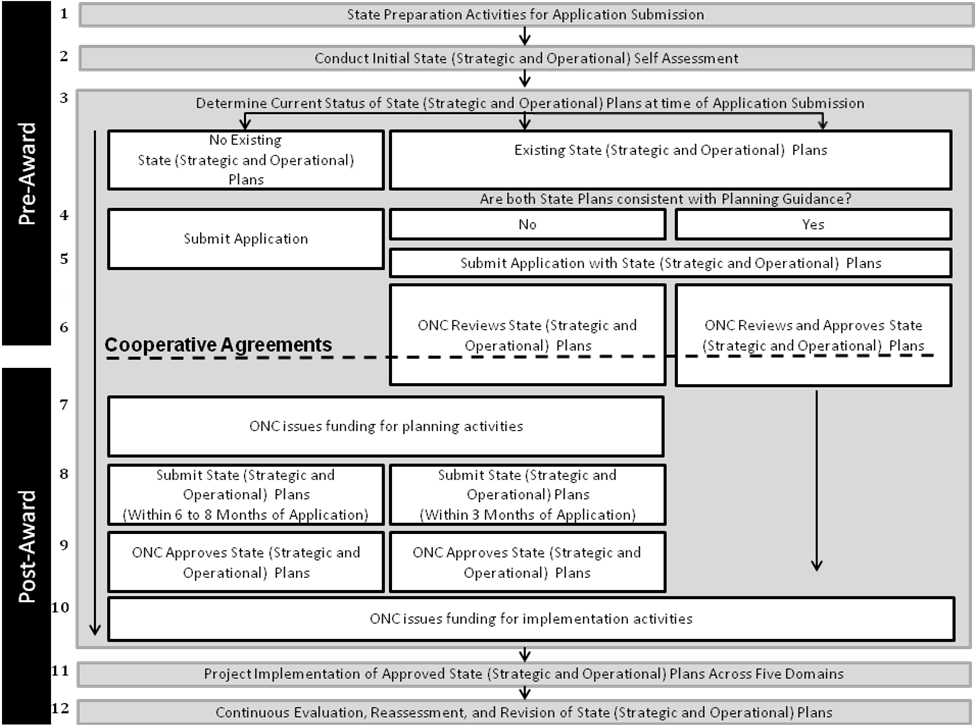
Figure E.1
Figure E.1 (above) describes the following activities:
Pre-Award Activities:
States will complete preparation activities in order to fill out their applications.
One of the preparation activities is the completion of an initial state self assessment.
In filling out applications, states will identify the current status of their state Strategic and Operational Plans.
As discussed in Section – I.E.1 states may have: no existing state Strategic and/or Operational Plans, existing state Strategic and/or Operational Plans that are not consistent with planning guidance, or existing state Strategic and Operational Plans that are consistent with planning guidance. The status of the state Strategic and Operational Plans, as well as the plans themselves must be included in the submission of the application.
Following the submission of the application and accompanying state Strategic and/or Operational Plans, ONC will review and if appropriate, will approve the submitted plans. The review and approval by ONC may occur prior to, during, and/or after the cooperative agreement is awarded.
Signing Cooperative Agreement Activity:
Following the submission of the application the states will enter into an appropriately tailored cooperative agreement with the federal government. If applicable, states may receive at Notice of Award prior to, during, or following the review and approval of their Strategic and/or Operational state plans.
Post-Award Activities:
States that do not have approved state Strategic and Operational Plans will be issued funding by ONC for state planning activities. States that have approved state Strategic and Operational Plans may be granted funding for continued planning activities. In addition, states with approved Strategic and Operational State plans will be permitted to forgo activities #8 and #9 and move immediately to activity #10, upon receipt of a Notice of Award.
States with no state Strategic or Operational Plans will have 6 to 8 months to submit their Plans. States with Strategic and Operational Plans that are not consistent with planning guidance will have 3 months to update and submit their Plans.
If not already completed in activity #5, ONC will approve state Strategic and Operational Plans.
Upon the completion of the state Strategic and Operational Plans, ONC will fund states’ implementation activities.
Funding will be used to conduct implementation activities in alignment with the approved state Strategic and Operational Plans, across the five domains associated with HIE.
In addition, states will conduct continuous evaluation, reassessment, and revision of their state Strategic and Operational Plans as needed and/or required.
-
Status
Materials for Submission
Type of Funds Available at Award
Application
Strategic Plan
Operational Plan
Planning
Implementation6
No Existing Strategic Plan
X
-
-
Yes
No
Existing Strategic Plan and/or Operational Plan that is not consistent with planning guidance
X
X
X (as applicable)
Yes
No
Existing Strategic and/or Operational Plan that is consistent with planning guidance
X
X
X
Yes
Yes
Table E.1
Once a state has submitted its application with the supporting Strategic and/or Operational Plans, ONC will review the Plans as one step in the overall application approval/response process. Recipients may receive awards prior to the Plans being approved. There could be adjustments required after the Plan evaluations are complete.
Not all states will meet all the criteria required of a Strategic or Operational Plan. ONC expects that most states will fall into one of the possible options outlined below. More detailed information regarding how to approach the application in each of these scenarios has been outlined above in Section I.E.2.
Status:
No Existing Strategic Plan:
States that submit applications with no existing Plans are eligible for award funding for Strategic and Operational Planning Activities
Existing Strategic Plan and/or Operational Plan that is not consistent with planning guidance:
Strategic Plan Only - States that submit applications with only Strategic Plans will be eligible for award and funding for Strategic and Operational Planning Activities.
Strategic Plan & Operational Plan - States that submit applications with both Strategic and Operational Plans will be eligible for award and funding for continued Strategic and Operational Planning activities.
Existing Strategic and/or Operational Plan that is consistent with planning guidance:
Additional funding for implementation activities will be awarded when the National Coordinator approves submitted implementation plans.
ONC will work closely with each recipient to identify where they stand along the continuum from planning through implementation. Additionally, ONC will provide ongoing program direction to assist states and SDEs in the planning and implementation of the five domains to enhance the effectiveness of state HIE initiatives.
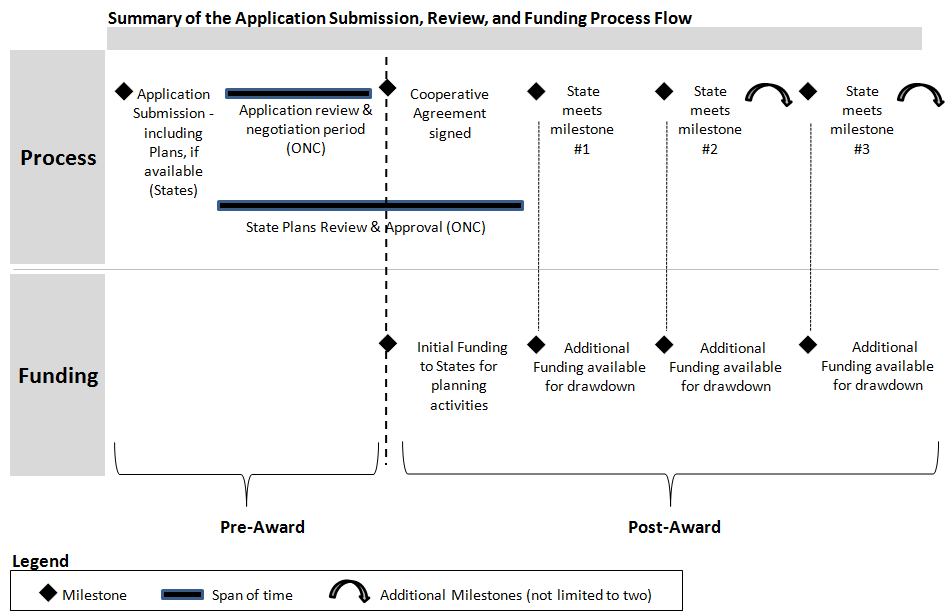
2.Application Submission, Review, and Funding Process
Below, Figure E.2 represents a high-level timeline of the Application Submission Review and Funding process flow. Immediately after a state submits an application that includes the accompanying Strategic and/or Operational Plans, review and negotiation period will take place between the state and ONC.
Implementation funding will become available once the National Coordinator has approved the State Plan.
Furthermore, additional funding available for drawdown will be determined by each state’s completion of agreed upon milestones and measures.
F.K
Figure E.2
ey Considerations & Challenges for HIE Implementations
1.Medicaid and Medicare Coordination
Throughout this program, recipients are required to ensure that all activities are consistent with and enable the implementation of the Medicaid and Medicare meaningful EHR use incentives. This shall be reflected in their governance structure, policy framework, HIE services, progress tracking and outcomes. State Plans under this program shall be consistent with and complementary to Medicaid and Medicare plans for the implementation of meaningful use incentives as they are developed.
2.Privacy and Security
Privacy and security of health information, including confidentiality, integrity and availability of information, are integral to fostering health information exchange. States and SDEs must establish how the privacy and security of an individual’s health information will be addressed, including the governance, policy and technical mechanisms that will be employed for health information exchange.
As applicable, recipients are expected to incorporate the privacy and security provisions of the ARRA, HIPAA Privacy Rule, HIPAA Security Rule, Confidentiality of Alcohol and Drug Abuse Patient Records Regulations, and the HHS Privacy and Security Framework into the State Strategic and Operational Plans. In addition, recipients are expected to collaborate on privacy and security policies with neighboring states to the extent necessary to facilitate HIE across state boundaries.
The ARRA includes specific privacy and security provisions related to security breach, restrictions and disclosures, sales of health information, consumer access, business associate obligations and agreements. Representative examples can be found in Appendix F.
The HIPAA Privacy Rule specifies permitted uses and disclosures and individual rights related to protected health information. These provisions are found at 45 CFR Part 160 and Part 164, Subparts A and E. For more details, please refer to: http://www.hhs.gov/ocr/privacy/hipaa/administrative/privacyrule/adminsimpregtext.pdf
The HIPAA Security Rule specifies a series of administrative, technical, and physical security procedures for covered entities to use to assure the confidentiality of electronic protected health information. These provisions are found at 45 CFR Part 160, and Part 164, Subparts A and C.C For more details, please refer to: http://www.hhs.gov/ocr/privacy/hipaa/administrative/privacyrule/adminsimpregtext.pdf.
The Confidentiality of Alcohol and Drug Abuse Patient Records Regulation (42 CFR Part 2) specifies confidentiality requirements for substance abuse treatment programs as defined by 42 CFR § 2.11 that are “federally assisted” as defined by 42 CFR § 2.12(b)). For more details, please refer to: http://www.hipaa.samhsa.gov.
The HHS Privacy and Security Framework establishes a single, consistent approach to address the privacy and security challenges related to electronic health information exchange through a network for all persons, regardless of the legal framework that may apply to a particular organization. The goal of this effort is to establish a policy framework for electronic health information exchange that can help guide the Nation’s adoption of health information technologies and help improve the availability of health information and health care quality. The principles have been designed to establish the roles of individuals and the responsibilities of those who hold and exchange electronic individually identifiable health information through a network. The principles are found in Appendix F.
To the extent that states anticipate exchanging health information with federal health care delivery organizations, such as the Department of Veterans Affairs (VA), Department of Defense (DoD), and the Indian Health Service (IHS), it will be important for the state to meet various federal requirements for protection of health data, as applicable.
As the program evolves over time, ONC plans to issue additional program guidance to further define the privacy and security requirements.
3.Interoperability
Adoption of HHS interoperability standards will be an important programmatic and policy goal, facilitated by ongoing federal and state efforts to advance interoperability. Additionally, ONC envisions that the Nationwide Health Information Network (NHIN) will continue to evolve and provide key capabilities to foster interoperability.
4.Consensus Definitions
In April 2008, ONC released a report providing consensus-based definitions of key health information technology terms in order to promote consistent usage of these terms during policy development, development of regulatory guidance, and implementation activities. The terms addressed in the report include Electronic Medical Record, Electronic Health Record, Personal Health Record, Health Information Exchange, Regional Health Information Organization and Health Information Organization. Please refer to the full report for a description of the methods used to develop these definitions, additional details for each definition, and for context-setting information about why consensus definitions are needed for health information technology activities. The full report is available by going to the link below:
http://healthit.hhs.gov/defining_key_hit_terms.
These terms shall be consistently applied throughout the application:
Records Terms
Electronic Medical Record (EMR) – an electronic record of health-related information regarding an individual that conforms to nationally recognized interoperability standards and that can be created, gathered, managed, and consulted by authorized clinicians and staff within one health care organization.
Electronic Health Record (EHR) – an electronic record of health-related information regarding an individual that conforms to nationally recognized interoperability standards and that can be created, managed, and consulted by authorized clinicians and staff across more than one health care organization.
Personal Health Record (PHR) – an electronic record of health-related information regarding an individual that conforms to nationally recognized interoperability standards and that can be drawn from multiple sources while being managed, shared, and controlled by the individual.
Network Terms
Health Information Exchange (HIE) - The electronic movement of health-related information among organizations according to nationally recognized standards. For the purposes of this program, organization is synonymous with healthcare providers, public health agencies, payors and entities offering patient engagement services (such as Patient Health Records) .
Regional Health Information Organization (RHIO) - A health information organization that brings together health care stakeholders within a defined geographic area and governs health information exchange among them for the purpose of improving health and care in that community.
Health Information Organization (HIO) - An organization that oversees and governs the exchange of health-related information among organizations according to nationally recognized standards.
G.Statutory Authority
The statutory authority for awards under this Funding Opportunity Announcement is contained in Section 3013 of the Public Health Service Act (PHSA), as amended by the American Recovery and Reinvestment Act of 2009 (ARRA), Division A—Appropriations Provisions, Subtitle B—Incentives for the Use of Health Information Technology. The statutory language of Section 3013 of the PHSA is included in Appendix A of this document.
II.Award Information
A.Summary of Funding
-
Type of Award:
Cooperative Agreement
Total Amount of Funding Available
$564,000,000
Award Floor7:
$4,000,000
Award Ceiling:
$40,000,000
Approximate Number of Awards8:
50
Program Period Length
4 years
Anticipated Project Start Date
January 15, 2010
ONC anticipates awarding not more than one cooperative agreement to fund activities in each state. Applications may cover a single state or consortium of more than one state. If a consortium applies, one state must take the lead role in applying for the cooperative agreement and in executing the work.
These cooperative agreements are intended to hasten the availability of the HIE capacity necessary for providers to qualify for the HITECH Act Medicare and Medicaid meaningful use incentive payments. To help the states and SDEs meet this critical need quickly, cooperative agreements will have a four-year project period, states will need to plan to use these funds in the most appropriate way possible to stay current and to build a sustainable HIE infrastructure that will succeed beyond the period of the cooperative agreement.
Funding, during the performance period, shall be contingent upon recipients’ ability to meet the matching requirements (outlined in further detail in Section III.B Matching Requirements), ability to meet agreed upon project milestones, compliance with other applicable statutory and regulatory requirements, and demonstrated organizational capacity to accomplish the program’s goals.
B.Type of Awards
Awards will be in the form of cooperative agreements to individual states, multi-state consortia, and SDEs. Terms and conditions for this cooperative agreement are found in Section VI.D. ONC will work closely with each recipient as planning and implementation progresses in a collaborative way.
During the approval process, appropriate project milestones and specific metrics will be agreed upon. As a project meets these milestones and measures, it will progress with additional funds available for drawdown. Funds will be made available to all applicants initially to address needed planning activities. (See Section IV.G.3. Other Funding Information – Performance-Based Funding). To obtain funding for implementation, the recipient must submit a Strategic and an Operational Plan and the plans must receive approval by the National Coordinator. ONC will evaluate the State Plans against the requirements outlined in Section I.D.3 and Appendix B.
ONC reserves the right to announce an additional round of funding in the future to provide for advanced implementation for those that have met all milestones in a timely manner within the project period, have distinguished themselves as leaders in the effort, and can provide leadership and document successes for national use.
III.Eligibility Information
A.Eligible Applicants
Either a state or a SDE may apply for cooperative agreements under this program. Multi-state efforts may also apply; however, one state or SDE must act as the responsible fiscal agent.9
Any entity applying for a cooperative agreement must satisfy the following criteria:
Be either:
A component of state government, or
A not-for-profit entity10.
Be designated by the state through a letter from the Governor (See Appendix D). For multi-state applications, a letter from the Governor (or equivalent) designating the partnering state or SDE must be received on behalf of each state participating in the proposed project.
The applicant must demonstrate that the program includes a multidisciplinary board or commission in an advisory or governing capacity with broad stakeholder representation that:
Represents a public/private partnership (Public and Private Sector Models for Governance can be found in Appendix H), and
Represents state and local needs, and
Retains the necessary authority to execute approved State Plans.11
One of the principal goals of the applicant organization is to use information technology to improve health care quality and efficiency through the authorized and secure electronic exchange and use of health information.
The applicant certifies that it has adopted nondiscrimination and conflict of interest policies that demonstrate a commitment to transparent, fair, and nondiscriminatory participation by stakeholders.
The state government (or governments for multi-state applications) has appointed a State Government HIT Coordinator who is a state official and will coordinate state government participation in HIE.
ONC will not accept more than one application from a single state or territory.
In the event that an application is not submitted on behalf of a state, by either the state or an SDE, ONC will encourage these states to seek inclusion in a neighboring state application, or to find a qualified not-for-profit organization to submit an application on its behalf. If there are geographic areas still not covered by activities of this program, ONC will consider other options to ensure activities are in place to meet the goal of nationwide HIE capacity.
B.Matching Requirements
ONC and Congress, as evidenced by the stated objectives in ARRA through the HITECH Act, recognize the urgency in expanding the use and availability of electronic health information on a nationwide scale. The HITECH Act requires a match to federal monies awarded to states beginning in fiscal year 2011. ONC and Congress also recognize that securing commitment and funding from other sources will strengthen a state’s sustainability plan and lead to greater success. Matching requirements can be provided through cash and/or in-kind contributions. The HITECH Act requires an increasing level of match for each year of the program:
-
Fiscal Year of Funding
Match Required
2010
None
2011 (begins Oct. 1, 2010)
$1 for each $10 federal dollars
2012 (begins Oct 1, 2011)
$1 for each $7 federal dollars
2013 (begins Oct 1, 2012)
$1 for each $3 federal dollars
1.Example Match Computation
For FY 2011, the applicant’s match requirement is $1 for every $10 federal dollars. In other words, for every ten dollars received in federal funding, the applicant must contribute at least one dollar in non-federal resources toward the program’s total cost. This “ten-to-one” ratio is reflected in the following formula that can be used to calculate minimum required match:
-
Federal Funds Requested =
10
Minimum
Match
Requirement
For example, if $100,000 in federal funds is requested for FY2011, then the minimum match requirement is $100,000/10 or $10,000. In this example the program’s total cost would be $110,000.
If the required non-federal share is not met by the award recipient, ONC will disallow any unmatched federal dollars. For the purposes of this program announcement, no match is required during fiscal year 2010. Beginning in fiscal year 2011, recipients will be required to match federal dollars as described in the table above. Demonstration of this match will be shown in quarterly financial reports. In preparing the application budget, applicants should consider these cost-sharing requirements and account for a match on their best estimate of expenditures for each period. For example, in year one of the project, there will be eight months where no match is required and four months where a 1-to-10 match is required. See table below for more information.
-
Ratio of Recipient to Federal Funding Share by Month
Feb
Mar
Apr
May
Jun
Jul
Aug
Sep
Fiscal Year Start Begins
Oct
Nov
Dec
Jan
FY 2010
$0
$0
$0
$0
$0
$0
$0
$0
1 to10
1 to10
1 to10
1 to10
FY 2011
1 to10
1 to10
1 to10
1 to10
1 to10
1 to10
1 to10
1 to10
1 to 7
1 to 7
1 to 7
1 to 7
FY 2012
1 to 7
1 to 7
1 to 7
1 to 7
1 to 7
1 to 7
1 to 7
1 to 7
1 to 3
1 to 3
1 to 3
1 to 3
FY 2013
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
1 to 3
C.Responsiveness and Screening Criteria
1.Application Responsiveness Criteria
Applications that do not meet the following responsiveness criteria will be administratively eliminated and will not be reviewed. The successful applicant will be an organization that meets the following criteria:
The application is the only application received from the state.
The applicant submitted a timely Letter of Intent as outlined in Section IV.C.1.
The applicant has met all applicable eligibility criteria as required by Section III.A – Eligible Applicants.
The applicant has submitted a complete application that includes all required components and attachments.
2.Application Screening Criteria
All applications will be screened to identify applications that do not meet criteria outlined below. These will be contacted by ONC and asked to revise their applications to meet the criteria; however, this could delay availability of funds.
In order for an application to be reviewed, it must meet the following screening requirements:
Applications must be submitted electronically via http://www.grants.gov by 5:00 p.m., Eastern Time, October 16, 2009.
The Project Narrative section of the Application must be double-spaced, on 8 ½” x 11” plain white papers with 1” margins on both sides, and a font size of not less than 11.
The Project Narrative must not exceed 40 pages. NOTE: The Letters of Intent and Support, and Resumes of Key Project Personnel are not counted as part of the Project Narrative for purposes of the 25-page limit.
If applicable, proof of not-for-profit status, or application for this status if the determination has not been made.
IV.Application and Submission Information
A.Award Administration
For purposes of this program, ONC has partnered with the Assistant Secretary for Preparedness and Response (ASPR) to act as ONC’s official grants management office. As such, applicants and recipients will work closely with ONC as well as ASPR. This will include pre-award activities such as application submission and review, and award notices. Post-award activities will include adjustments to cooperative agreements, budget support, and technical support using Grantsolution.gov.
B.Address to Request Application Package
Application materials can be obtained from http://www.grants.gov or http://www.GrantSolutions.gov.
If you have difficulty obtaining the application materials from the sites above, please email ONC at StateHIEgrants@hhs.gov.
Please note that ONC is requiring applications for all announcements to be submitted electronically through http;//www.grants.gov. The Grants.gov registration process can take several days. If your organization is not currently registered with http://www.grants.gov, please begin this process immediately. For assistance with http://www.grants.gov, please contact them at support@grants.gov or 1-800-518-4726 between 7 a.m. and 9 p.m. Eastern Time. At http://www.grants.gov, applicants will be able to download a copy of the application packet, complete it off-line, and then upload and submit the application via the Grants.gov website.
Applications submitted via http://www.grants.gov:
You may access the electronic application for this program on http://www.grants.gov. Applicants must search the downloadable application page by the Funding Opportunity Number (EP-HIT-09-001) or CFDA number (93.719).
At the http://www.grants.gov website, applicants will find information about submitting an application electronically through the site, including the hours of operation. ONC strongly recommends that you do not wait until the application due date to begin the application process through http://www.grants.gov because of the time delay.
All applicants must have a Dun and Bradstreet (D&B) Data Universal Numbering System (DUNS) number and register in the Central Contractor Registry (CCR). Applicants should allow a minimum of five days to complete the CCR registration.
Applicants must submit all documents electronically, including all information included on the SF424 and all necessary assurances and certifications.
Prior to application submission, Microsoft Vista and Office 2007 users should review the grants.gov compatibility information and submission instructions provided at http://www.grants.gov (click on “Vista and Microsoft Office 2007 Compatibility Information”).
Applications must comply with any page limitation requirements described in this Program Announcement.
After applications are submitted electronically, applicants will receive an automatic acknowledgement from http://www.grants.gov that contains a grants.gov tracking number. ONC will retrieve applications form from grants.gov.
After ONC retrieves applications form grants.gov, a return receipt will be emailed to the applicant contact. This will be in addition to the validation number provided by grants.gov.
Each year organizations registered to apply for federal awards through http://www.grants.gov will need to renew their registration with the Central Contractor Registry (CCR). Applicants can register with the CCR online and it will take about 30 minutes (http://www.ccr.gov).
Applicants must have a Grantsolutions.gov account to apply for this opportunity. Registration and user information can be found at http://www.grantsolutions.gov.
C.Content and Form of Application Submission
1.Letter of Intent
Applicants are required to submit a letter of intent (electronically or by mail) to apply for this funding opportunity to assist ONC in planning for the independent review process. For multi-state applications, only one letter of intent should be submitted. This letter should be submitted by the state or SDE that will act as the applicant on behalf of all states involved in the proposed project. The letter of intent should be no longer than 5 pages. The letter of intent must be received by 5:00 pm, EST, September 11, 2009. The required content for this letter is included in Appendix C. Letters of intent should be sent to:
David Blumenthal MD, MPP
National Coordinator for Health Information Technology
Department of Health and Human Services
200
Independence Avenue, S.W.
Washington, DC 20201
Tel: (202) 690-7151
StateHIEgrants@hhs.gov
2.DUNS Number
The Office of Management and Budget (OMB) requires applicants to provide a Dun and Bradstreet (D&B) Data Universal Numbering System (DUNS) number when applying for federal grants or cooperative agreements on or after October 1, 2003. It is entered on the SF 424. It is a unique, nine-digit identification number, which provides unique identifiers of single business entities. The DUNS number is free and easy to obtain, though applicants should allow a minimum of five days to complete the CCR registration.
Organizations can receive a DUNS number at no cost by calling the dedicated toll-free DUNS Number request line at 1-866-705-5711 or by using this link to access a guide: https://www.whitehouse.gov/omb/grants/duns_num_guide.pdf.
3.Tips for Writing a Strong Application
Tips for writing a strong application can be found at HHS’ GrantsNet site at http://www.hhs.gov/grantsnet/AppTips.htm.
4.Project Abstract
Applicants shall include a one-page abstract (no more than 500 words) of the application. This abstract is often distributed to provide information to the public and Congress and represents a high-level summary of the project. Applicants should prepare a clear, accurate, concise abstract that can be understood without reference to other parts of the application and which gives a description of the proposed project, including: the project’s goal(s), objectives, overall approach (including target population and significant partnerships), anticipated outcomes, products, and duration. Detailed instructions for completing the summary/abstract are included in Appendix L of this document.
The Project Abstract must be double-spaced with a font size of not less than 11 point.
The applicant shall place the following information at the top of the Project Abstract (this information is not included in the 500 word maximum):
Project Title
States/territories included in the application
Applicant Name
Address
Contact Name
Contact Phone Numbers (Voice, Fax)
E-Mail Address
Web Site Address, if applicable
Congressional districts within your service area
Brief explanation of where the state is in achieving statewide HIE among healthcare providers
The Project Abstract must include a brief description of the proposed cooperative agreement, how the activities support and will enhance HIE services across all health care and public health stakeholders, the current status of the state’s efforts, the need(s) to be met with the funds, the design and scope of the state’s plan.
5.Project Narrative
The Project Narrative is the most important part of the application, since it will be used as the primary basis to determine whether or not the application meets the minimum requirements for funding. The Project Narrative must provide a detailed picture of the current state of HIE in the state (and at the multi-state level, if applicable) and must describe the needs of specific geographic areas of the state to achieve greater availability and use of electronic health information exchange. The Project Narrative is in addition to the outlined State Plan (Strategic and Operational). The narrative must provide the reader with an understanding of the state’s current efforts and what activities are planned through the State HIE Program to implement health information exchange across the state or region. As appropriate, applicants should reference the pathway to HIE and the five critical domains discussed above.
The Project Narrative must be double-spaced, on 8 ½” x 11” papers with 1” margins on both sides, and a font size of not less than 11. Smaller font sizes may be used to fill in the Standard Forms and Sample Formats. The suggested length for the Project Narrative is 25 to 40 pages; 40 pages is the maximum length allowed. ONC will not accept applications with a Project Narrative that exceeds 40 pages. The State Plans (Strategic and Operational Plans), Governor’s Designation Letter, Project Abstract, Letters of Commitment, and Resumes of Key Personnel are not counted as part of the Project Narrative for purposes of the 40-page limit, but all of the other sections noted below are included in the limit.
The components of the Project Narrative counted as part of the 40 page limit include:
Current State
Proposed Project Strategy
Required Performance Measures
Project Management
Evaluation
Organizational Capability Statement
The Project Narrative is a critical part of the application as it will be used as the primary basis to determine whether or not the application meets the minimum requirements for funding under the HITECH Act. The Project Narrative should provide a clear and concise description of the project. ONC recommends that the project narrative include the following components:
a)Current State
In this section applicants shall:
Discuss and determine the current status of the state’s progress in achieving statewide HIE among healthcare providers, including:
Electronic eligibility and claims transactions
Electronic prescribing and refill requests
Electronic clinical laboratory ordering and results delivery
Electronic public health reporting (immunizations, notifiable laboratory results)
Quality reporting capabilities
Prescription fill status and/or medication fill history
Clinical summary exchange for care coordination and patient engagement.
Describe the progress and status of the state in its project planning and implementation as described in Section I.E.1., Self-Assessment of the State’s Current Status.
b)Proposed Project Summary
This section should provide a clear and concise description of activities funded by the cooperative agreement to develop, finalize and maintain Strategic and Operational plans to increase the extent of electronic information exchange for the HIE program objectives. It is not expected to be a summary of a state’s existing state plans. Applicants must articulate the rationale for the overall approach to the project. Also note any major barriers anticipated to be encountered and how the project will be able to overcome those barriers. The project summary should include all portions required but applicants may frame their answers according to their current status (whether the state has an existing plan or intends to develop or finalize one using federal funds). It is expected that those applicants with plans will have more fully developed and final responses while those without applications may address intended approaches to be used. The proposed summary shall include:
For states without existing state plans at the time of application, a description of the approach the applicant proposes to develop and finalize such a plan.
For states with existing state plans at time of application, a description of the approach the applicant proposes to implement the plan including the mechanisms to overcome obstacles and a realistic and achievable high-level project plan and timeline.
A discussion of approach to be employed to ensure compliance with the Privacy and Security requirements for Health IT as outlined in Section I.F.2., Privacy and Security.
A description of the proposed communications strategy with key stakeholders and the health community.
A description of how the applicant plans to involve community-based organizations in a meaningful way in the planning and implementation of the proposal project. This section should also describe how the proposed intervention will target medically underserved populations, and the needs of special populations including newborns, children, youth, including those in foster care, the elderly, persons with disabilities, Limited English Proficiency (LEP) persons, persons with mental and substance use disorders, and those in long term care.
A discussion of how the interests of the stakeholders below will be considered and incorporated into planning and implementation activities.
Health care providers, including providers that provide services to low income and underserved populations
Health plans
Patient or consumer organizations that represent the population to be served
Health information technology vendors
Health care purchasers and employers
Public health agencies
Health professions schools, universities and colleges
Clinical researchers
Other users of health information technology such as the support and clerical staff of providers and others involved in the care coordination of patients
Additionally, for those submitting collaborative applications (multi-state/territory), a discussion that:
Demonstrates that the application represents the best interest of each state or territory involved in the consortium.
Documents how financial accountability will be assured, so that risks and challenges faced by one member of the collaborative do not impede the progress of another member and develop a reporting mechanism that tracks expenditures and activities by state.
Describes how governance standards will be met, to include governance structures at the state/territory level that is represented within a collaborative governance structure.
Documents how financial accountability will be assured, so that risks and challenges faced by one member of the collaborative do not impede the progress of another member.
Ensures that sufficient funds will be available to each state/territory for planning at the state level.
c)Required Performance Measures and Reporting
Reporting and Performance Measures are required for applicants requesting funding for planning or implementation activities. Reporting Requirements must be submitted by applicants requesting funding for planning and/or implementation activities. Once a recipient has entered into implementation activities, the Performance Measures become ongoing requirements.
The applicant shall provide detailed information in the application about the methodologies, tools, and strategies they intend to use to collect all data, including the reporting requirements and performance measures, for the project to satisfy the reporting requirements of this program and the Government Performance Reporting Act of 2003. Other performance measures specific to ARRA reporting are required and provided in Appendix G. ARRA reporting requirements will also be included in the Notice of Award. The performance measures will be used as part of the state and/or national program evaluation. As the program evolves, additional requirements may be provided through program guidance.
Specific reporting requirements, performance and evaluation measures and methods to collect data and evaluate project performance will be provided at a later date in program guidance and through technical assistance, prior to award of cooperative agreements. These measures will include those related to the following domains: governance, finance, technical infrastructure, business and technical operations, and legal/policy. The core set of reporting requirements and performance measures enables states to monitor their own progress, and when aggregated across recipients, provides ONC with a national view of progress across the program. The core set of reporting requirements and performance measures includes but are not limited to:
Reporting Requirements
(Required for those requesting funding for planning and/or implementation activities)
Governance
What proportion of the governing organization is represented by public stakeholders?
What proportion of the governing organization is represented by private sector stakeholders?
Does the governing organization represent government, public health, hospitals, employers, providers, payers and consumers?
Does the state Medicaid agency have a designated governance role in the organization?
Has the governing organization adopted a strategic plan for statewide HIT?
Has the governing organization approved and started implementation of an operational plan for statewide HIT?
Are governing organization meetings posted and open to the public?
Do regional HIE initiatives have a designated governance role in the organization?
Finance
Has the organization developed and implemented financial policies and procedures consistent with state and federal requirements?
Does organization receive revenue from both public and private organizations?
What proportion of the sources of funding to advance statewide HIE are obtained from federal assistance, state assistance, other charitable contributions, and revenue from HIE services?
Of other charitable contributions listed above, what proportion of funding comes from health care providers, employers, health plans, and others (please specify)?
Has the organization developed a business plan that includes a financial sustainability plan?
Does the governance organization review the budget with the oversight board on a quarterly basis?
Does the recipient comply with the Single Audit requirements of OMB?
Is there a secure revenue stream to support sustainable business operations throughout and beyond the performance period?
Technical Infrastructure
Is the statewide technical architecture for HIE developed and ready for implementation according to HIE model(s) chosen by the governance organization?
Does statewide technical infrastructure integrate state-specific Medicaid management information systems?
Does statewide technical infrastructure integrate regional HIE?
What proportion of healthcare providers in the state are able to send electronic health information using components of the statewide HIE Technical infrastructure?
What proportion of healthcare providers in the state are able to receive electronic health information using components of the statewide HIE Technical infrastructure?
Business and Technical Operations
Is technical assistance available to those developing HIE services?
Is the statewide governance organization monitoring and planning for remediation of HIE as necessary throughout the state?
What percent of health care providers have access to broadband?
What statewide shared services or other statewide technical resources are developed and implemented to address business and technical operations?
Legal/Policy
Has the governance organization developed and implemented privacy policies and procedures consistent with state and federal requirements?
How many trust agreements have been signed?
Do privacy policies, procedures and trust agreements incorporate provisions allowing for public health data use?
Performance Measures
The following measures are applicable to the implementation phase of the cooperative agreement. They are an initial set of measures intended to give a state specific and national perspective on the degree of provider participation in HIE enabled state level technical services and the degree to which pharmacies and clinical laboratories are active trading partners in HIE. E-prescribing and laboratory results reporting are two of the most common types of HIE within and across states.
Percent of providers participating in HIE services enabled by statewide directories or shared services.12
Percent of pharmacies serving people within the state that are actively supporting electronic prescribing and refill requests.
Percent of clinical laboratories serving people within the state that are actively supporting electronic ordering and results reporting.
Recipients will also be required to report on additional measures that will indicate the degree of provider participation in different types of HIE particularly those required for meaningful use.
Future areas for performance measures that will be specified in program guidance will include but are not limited to: providers’ use of electronic prescribing, exchange of clinical summaries among treating providers, immunization, quality and other public health reporting and eligibility checking.
d)Project Management
This section should include a clear delineation of the roles and responsibilities of project staff, consultants and partner organizations, and how they will contribute to achieving the project’s objectives and outcomes. It should specify who would have day-to-day responsibility for key tasks such as: leadership of project; monitoring the project’s on-going progress, preparation of reports, and communications with other partners and ONC. It should also describe the approach that will be used to monitor and track progress on the project’s tasks and objectives.
e)Evaluation
This section should describe the method(s), techniques and tools that will be used to track and maintain project information expected to be required for the state to conduct a self-evaluation of the project and to inform a national program-level evaluation.
f)Organizational Capability Statement
Each application shall include an organizational capability statement. The organizational capability statement should describe how the applicant agency (or the particular division of a larger agency that will have responsibility for this project) is organized, the nature and scope of its work and/or the capabilities it possesses. It should also include the organization’s capability to sustain some or all project activities after federal financial assistance has ended. It must define who is considered key staff and the applicant must provide resumes for each key staff member in the attachments to the application, which are not included in the page limitation.
This description should cover capabilities of the applicant agency, such as any current or previous relevant experience and/or the record of the project team in preparing cogent and useful reports, publications, and other products. If appropriate, include in the attachments an organization chart showing the relationship of the project to the current organization, which will not count toward the page will limit. Also include information about any contractual organization(s) that will have a significant role(s) in implementing project and achieving project goals.
6.Required Plans
If, at the time of application, the applicant has a state plan (Strategic or Operational) that is either consistent or not consistent with planning guidance in this document, it should be included with this application.
Applicants that have plans that are not consistent with the planning guidance may take the time during application period to revise their Strategic and Operational Plans to be consistent with planning guidance, if they choose. The applicant should indicate if the State Plan submitted with this application is submitted for official approval by the National Coordinator.
7.Collaborations and Letters of Commitment from Key Participating Organizations and Agencies
The applicant shall fully describe the current relationships established to meet the State’s HIE goals. If there are relationships that have yet to be formalized, provide a plan for engaging these groups. The applicant must also include, in an attachment to the application, a copy of the interagency agreement (or similar document) that outlines the parameters of such relationships. At a minimum this section must explain the demonstrated commitment on the part of the state government and how the state and project coordinate with critical stakeholders.
Include confirmation of the financial or in-kind commitments to the project (should it be funded) made by key collaborating organizations and agencies in this part of the application. Any organization that is specifically named to have a significant role in carrying out the project should be considered an key collaborating organization and a letter of support should be included for each. For applications submitted electronically via grants.gov, signed letters of commitment should be scanned and included as attachments. These letters should not be considered as part of the 25 page limit. A template for these letters can be found in Appendix E.
8.Budget Narrative/Justification
All applicants are required to outline proposed costs that support all project activities in the Budget Narrative/Justification. The application must include the allowable activities that will take place during the funding period and outline the estimated costs that will be used specifically in support of the program. Costs are not allowed to be expended until the start date listed in the Notice of Grant Award. All costs must be allowable, allocable, reasonable and necessary under the applicable OMB Cost Circular: www.whitehouse.gov/omb/circulars (Circular A-87 for States and Circular A-122 for SDEs) and based on the programmatic requirements for administering the program as outlined in ARRA.
Prior to the application due date, and after submission of the required letter of intent, eligible applicants will be provided an allocation amount for the proposed project period. This figure will be determined as described in Section G.2 – Other Funding Information, below. This amount plus required match should be the total of all allowable project costs for the four year project period. Applicants are required to submit a one year budget for each of the four years of the project period.
Applicants are suggested to use the format included as Appendix K of this Funding Opportunity Announcement. Applicants are also encouraged to pay particular attention to Appendix J, which provides an example of the level of detail sought. A combined multi-year Budget Narrative/Justification, as well as a detailed Budget Narrative/Justification for each year of potential grant funding is required. Instructions are also included in Appendix I as they pertain to completing the SF 424.
D.Submission Dates and Times
Letters of Intent to Apply must be submitted electronically or by mail, no later than 5:00 p.m. Eastern Standard Time on August 31, 2009. For those applicants who are not a state agency, a Governor’s Designation letter on official letterhead must be attached to the Letter of Intent. Formats for both documents are included in Appendices D and C, respectively. Information on where to submit the Letter of Intent can be found at Section IV.C.1.
Applications must be submitted via grants.gov no later than 5:00 p.m. EST on October 16, 2009.
Applications that fail to meet the application due date will not be reviewed and will receive no further consideration.
Grants.gov will automatically send applicants a tracking number and date of receipt verification electronically once the application has been successfully received and validated in grants.gov. After the Office of Grants Management retrieves the application form from grants.gov, a return receipt will be emailed to the applicant contact. This will be in addition to the validation number provided by grants.gov.
E.Intergovernmental Review
This program is excluded from Executive Order 12372.
F.Funding Restrictions
Applicants responding to this announcement may request funding for a project period of up to four years.
ONC will negotiate with applicants regarding allowable activities consistent with the yet-to-be developed Medicare/Medicaid “meaningful use” definition. ONC reserves the right to not award a cooperative agreement to any applicant that proposes activities that are not aligned with the goals and vision of enabling standards-based HIE in support of meaningful use and a high performance health care system.
Funds under this announcement cannot be used for the following purposes:
To supplant or replace current public or private funding.
To supplant on-going or usual activities of any organization involved in the project.
To purchase or improve land, or to purchase, construct, or make permanent improvements to any building except for minor remodeling.
To reimburse pre-award costs.
Funds are to be used in a manner consistent with program policies developed by ONC and within allowable budget categories outlined in Appendix I and J. Allowable administrative functions/costs include:
Usual and recognized overhead, including indirect rates for all consortium organizations that have an approved indirect cost rate by a federal cognizant agency.
2% of total project costs must be included in the budget for project evaluation.
G.Other Funding Information
1.Project Period
The four-year project period is intended to allow recipients time to complete the goals of the program. However, applicants are strongly encouraged to plan projects and budgets that accomplish most of the project goals and milestones within the first two years of the project period to best enable HIE capacity.
Funding decisions will be made based on a combination of formulaic allocations and needs-based assessment. More specific information will be forthcoming, but a general description of the process is below.
2.Funding Formula
Base Allocation: Each state, the District of Columbia, and the Commonwealth of Puerto Rico will be given an equal base amount of $4,000,000. American Samoa, Guam, the Northern Mariana Islands, and the Virgin Islands will each receive a base amount adjusted to reflect their population. Given the complexity, urgency, and importance of the work associated with achieving HIE services to reach all health care providers in the territories, we strongly encourage each of the territories to team with a state for the purposes of this cooperative agreement. For those that apply using a multi-state approach, the base amount will be adjusted to reflect the efficiencies of shared services.
Equity Adjustments: For states and the District of Columbia: Additional funds will be added to this base amount to account for differences in existing health care delivery environment. These additional funds will be determined by formula using the following equity factors – number of primary care physicians, number of short-term (acute) care hospitals, state population, and indicators of rural and underserved areas.
Following are the sources of information to be used for these equity adjustments along with the associated weights for each:
PCP Populations –The Robert Graham Center, as an extract of the American Medical Association’s master data file. Primary care physicians, for the purpose of this funding formula include MD/DO family physicians, general internists, and pediatricians. (40% of total allocation).
Short-Term (Acute) Care Hospital –The CMS Point of Service file, identifying the number of acute care and pediatric facilities in each state. (30% of total allocation).
Medically Underserved and Rural Providers –The CMS Point of Service file, identifying the Federally Qualified Health Center, and Rural Health Clinics in each state. (25% of total allocation).
State Population – 2000 Census estimates for 2008, used to determine the population for each state. (5% of the total allocation).
Needs-Based Adjustments: ONC will allocate 10% of the total funds available using information provided by the applicant regarding their historic investment in HIE as required in the Letter of Intent (see Appendix C, Required Format for Letter of Intent to Apply). States, the District of Columbia, and territories will be ranked on a scale of 1-3 based on historic investment, with a lower level of investment indicating a higher need for HIE grant funding.
Base Allocation + Equity Adjustments + Needs-Based Adjustments = Full Cooperative Agreement Award Amount
Unobligated funds at the end of the budget/project period are restricted and remain in the account for future disposition. Unobligated funds are those reported on the final Financial Status Report (SF-269), which is required to be submitted after the end of the budget/project period.
3.Performance-Based Funding
The performance and other reports submitted by award recipients will help to determine the project’s progress. Special conditions will be placed on each cooperative agreement that divides total funding among major milestones and meeting specific metrics for the program. For example, those recipients who do not have State Plans may drawdown funds for planning purposes; when the plan is complete and approved, the recipient will be able to drawdown additional funds related for implementation. Other milestones may include the initiation and completion and/or certain implementation activities of HIE Stages. Specific measures may include the HIE services that are available to providers.
4.Indirect Costs
Applicants should reference their approved indirect costs rates for any management and administrative needs while budgeting. ONC will not reimburse indirect costs unless the recipient has an approved indirect cost rate covering the applicable activities and period. Applicants are encouraged to consider budgeting for lower indirect cost rates in an effort to direct more resources toward project goals.
H.Other Submission Requirements
Applicants are required to attend the State HIE Leadership Training and the State HIE Forum, supported by ONC. The submitted budget must reflect funds allocated for travel for two people to attend each event for two days each year of the project period. One will be held in Washington, D.C. and one will be in Chicago, Illinois. Applicant’s attendance is an annual requirement.
I.Summary of Required Attachments
Copy of Letter of Intent, as previously submitted (Appendix C).
Letter designating the component of state government that will apply or a private entity as the SDE (Appendix D).
Letters of Support from critical stakeholders (Appendix E).
Not-for-profit certification or pending application (for State Designated Entities).
State Plan (if available).
V.Application Review Information
A.Criteria
A panel that may include both expert peer reviewers and federal staff will review each application that meets the responsiveness and screening criteria in Section III.C, 1 and 2. The purpose of this review is to determine if the approach, strategy, and any provided state plans are aligned with program requirements, not as a competitive means of comparing applications. The detailed results of this review will be shared with the applicant upon request. Additionally, the review results will form the basis for development of the programmatic terms and conditions of the cooperative agreement. These terms and conditions will outline the necessary milestones that must be met to continue receiving funds. Lastly, the review results will assist Project Officers in their collaborative discussions with the applicant regarding needed changes and for continued collaboration with recipients.
Each of the following items within each section will be assessed on a three point scale. A score of one means that the application has not met the requirements; a score of two means that the application has met requirements; a score of three means that the application has exceeded requirements. If an applicant fails to address the item, a score of zero will be given.
Applications will be reviewed for the following items:
Current State and Gap Analysis
Determination of current status of the state’s level of maturity as currently described in Section I.D.1.a, The Stages of HIE.
Determination of the progress and status of the state in its project planning and implementation as described in Section I.E.1., Self - Assessment of the State’s Current Status.
Proposed Strategy
For states without existing State Plans at time of application, an assessment of the strategy the applicant proposes to develop and finalize such a plan.
For states with existing State Plans at time of application, an assessment of the strategy the applicant proposes to implement the plan including:
The approaches to overcome obstacles described.
Whether the proposed project plan and timelines are realistic and achievable.
A determination of the alignment of the application’s description of the Privacy and Security requirements for Health IT as required by Section I.F.2., Privacy and Security.
An assessment of the proposed communications strategy with key stakeholders and the health community.
An assessment of the strategy to incorporate special target populations and organizations, as described in Project Narrative section.
An assessment of whether the application demonstrates how the interests of the stakeholders below will be considered and incorporated into planning and implementation activities.
Health care providers, including providers that provide services to low income and Underserved populations
Health plans
Patient or consumer organizations that represent the population to be served
Health information technology vendors
Health care purchasers and employers
Public health agencies
Health professions schools, universities and colleges
Clinical researchers
Other users of health information technology such as the support and clerical staff of providers and others involved in the care coordination of patients
For those submitting collaborative applications (multi-state/territory), an assessment of whether the applicant organization:
Demonstrates that the application represents the best interest of each state or territory involved in the consortium.
Documents how financial accountability will be assured, so that risks and challenges faced by one member of the collaborative do not impede the progress of another member and develop a reporting mechanism that tracks expenditures and activities by state.
Describes how governance standards will be met, to include governance structures at the state/territory level that is represented within a collaborative governance structure.
Documents how financial accountability will be assured, so that risks and challenges faced by one member of the collaborative do not impede the progress of another member.
Ensures that sufficient funds will be available to each state/territory for planning at the state level.
Project Management
An assessment of whether the proposed staffing of the project is adequate to achieve the stated goals and to develop and/or implement State Plans.
An assessment of whether the proposed strategy for project management is adequate to ensure progress and the ability to meet the stated goals and/or implement State Plans in a timely and effective manner.
Evaluation and Performance Measures
An assessment of the quality and thoughtfulness of the techniques to be employed by the applicant to track and maintain project information and metrics.
Organizational Capability Statement
An assessment of the organizational capability and background to carry out the goals and requirements of the program.
An assessment of the organization’s ability to sustain the project after federal assistance ends.
Budget Narrative/Justification
An assessment of the proposed costs for allocability, reasonableness and allowability of costs.
An assessment of the proposed costs’ alignment with ONC program and proposed project goals.
B.Review and Selection Process
An independent review panel of at least three individuals will evaluate applications that pass the screening and meet the responsiveness criteria, if applicable. These reviewers will be experts in their field, and will be drawn from academic institutions, non-profit organizations, state and local government, and federal government agencies. Based on the Application Review Criteria as outlined under Section V.A, the reviewers will comment on and score the applications, focusing their comments and scoring decisions on the identified criteria.
Final award decisions will be made by The National Coordinator for Health Information Technology. In making these decisions, The National Coordinator for Health Information Technology will take into consideration: recommendations of the review panel; reviews for programmatic and grants management compliance; the reasonableness of the estimated cost to the government considering the available funding and anticipated results; and the likelihood that the proposed project will result in the benefits expected.
Applicants have the option of omitting from the application specific salary rates or Social Security Numbers for individuals specified in the application budget.
VI.Award Administration Information
A.Award Notices
Each applicant will receive notification of the outcome of the review process outlined in Section V.A, including whether the application was selected for funding. The authorized representative of the state or SDE selected for funding will be required to accept the terms and conditions placed on their application before funding can proceed. Letters of notification acknowledge that an award was funded, but do not provide authorization for the applicant to begin performance and expend funds associated with the award until the start date of the award as indicated in the notice. Applicants may request a summary of the expert committee’s assessment of the application’s merits and weaknesses.
The Notice of Grant Award (NGA) contains details on the amount of funds awarded, the terms and conditions of the cooperative agreement, the effective date of the award, the budget period for which support will be given, the required match to be provided, and the total project period timeframe. This NGA is then signed by the ONC Grants Management Officer, sent to the applicant agency’s Authorized Representative, and will be considered the official authorizing document for this award. It will be sent to applicants prior to the start date of this program January 15, 2010.
Successful applicants will receive an electronic NGA from ASPR. This is the authorizing document notifying the applicant of the award from the U.S. Assistant Secretary for Preparedness and Response authorizing official, Officer of Grants Management, and the ASPR Office of Budget and Finance. Unsuccessful applicants are notified within 30 days of the final funding decision and will receive a disapproval letter via e-mail or U.S. mail.
B.Administrative and National Policy Requirements
The award is subject to HHS Administrative Requirements, which can be found in 45CFR Part 74 and 92 and the Standard Terms and Conditions implemented through the HHS Grants Policy Statement located at http://www.hhs.gov/grantsnet/adminis/gpd/index.htm.
1.HHS Grants Policy Statement
ONC awards are subject to the requirements of the HHS Grants Policy Statement (HHS GPS) that are applicable to the grant/cooperative agreement based on recipient type and purpose of award. This includes, as applicable, any requirements in Parts I and II of the HHS GPS that apply to the award, as well as any requirements of Part IV. The HHS GPS is available at http://www.hhs.gov/grantsnet/adminis/gpd/ . The general terms and conditions in the HHS GPS will apply as indicated unless there are statutory, regulatory, or award-specific requirements to the contrary (as specified in the Notice of Award).
a)Records Retention
Recipients generally must retain financial and programmatic records, supporting documents, statistical records, and all other records that are required by the terms of a grant, or may reasonably be considered pertinent to a grant, for a period of three years from the date the annual FSR is submitted. For awards where the FSR is submitted at the end of the competitive segment, the three-year retention period will be calculated from the date the FSR for the entire competitive segment is submitted. Those recipients must retain the records pertinent to the entire competitive segment for three years from the date the FSR is submitted. See 45 CFR 74.53 and 92.42 for exceptions and qualifications to the three-year retention requirement (e.g., if any litigation, claim, financial management review, or audit is started before the expiration of the three-year period, the records must be retained until all litigation, claims, or audit findings involving the records have been resolved and final action taken). Those sections also specify the retention period for other types of grant-related records, including indirect cost proposals and property records. See 45 CFR 74.48 and 92.36 for record retention and access requirements for contracts under grants.
C.Reporting
All reporting requirements will be provided to successful applicants, adherence to which is a required condition of any award. In general, the successful applicant under this guidance must comply with the following reporting and review activities:
1.Audit Requirements
The recipient shall comply with audit requirements of Office of Management and Budget (OMB) Circular A-133. Information on the scope, frequency, and other aspects of the audits can be found on the Internet at http://www.whitehouse.gov/omb/circulars.
2.Financial Status Reports
The recipient shall submit an annual Financial Status Report. An SF-269 financial status report is required within 90 days of the end of each budget and project period. The report is an accounting of expenditures under the project that year. More specific information on this reporting requirement will be included in the Notice of Grant Award.
3. Progress Reports
Progress Reports will be evaluated by ONC and are required on a semi-annual basis. ONC will provide required additional reporting instructions after awards are made.
As component of regular reporting, recipients will be required to detail expenditure information that reflect spending on developing a statewide governance and policy framework and developing HIE capacity with the state. Exceptions to this reporting requirement include activities related to the development of the state’s Strategic Plan and statewide shared services and directories that meet HHS adopted standards. Format and guidance for this requirement will be included in future program guidance.
4.ARRA-Specific Reporting
Quarterly Financial and Programmatic Reporting: Consistent with the Recovery Act emphasis on accountability and transparency, reporting requirements under Recovery Act programs will differ from and expand upon HHS’s standard reporting requirements for grants. In particular, section 1512(c) of the Recovery Act sets out detailed requirements for quarterly reports that must be submitted within 10 days of the end of each calendar quarter. Receipt of funds will be contingent on meeting the Recovery Act reporting requirements.
The information from recipient reports will be posted on a public website. To the extent that funds are available to pay a recipient’s administrative expenses, those funds may be used to assist the recipient in meeting the accelerated time-frame and extensive reporting requirements of the Recovery Act.
ONC may post information on the public website that identifies recipients that are delinquent in their reporting requirements. Additionally, recipients who do not submit required reports by the due date will not be permitted to drawdown funds thereafter, during the pendency of the delinquency, and may be subject to other appropriate actions by ONC, including, but not limited to, restrictions on eligibility for future ONC awards, restrictions on draw-down on other HHS awards, and suspension or termination of the Recovery Act award.
ONC may provide a standard form or reporting mechanism that recipients would be required to use. Additional instructions and guidance regarding required reporting will be provided as they become available. For planning purposes, however, all applicants shall be aware that the Recovery Act section 1512(c) provides as follows:
Recipient Reports: Not later than 10 days after the end of each calendar quarter, each recipient that received recovery funds from a federal agency shall submit a report to that agency that contains—
(1) The total amount of recovery funds received from that agency;
(2) The amount of recovery funds received that were expended or obligated to projects or activities; and
(3) A detailed list of all projects or activities for which recovery funds were expended or obligated, including--
(A) The name of the project or activity;
(B) A description of the project or activity;
(C) An evaluation of the completion status of the project or activity;
(D) An estimate of the number of jobs created and the number of jobs retained by the project or activity; and
(E) For infrastructure investments made by State and local governments, the purpose, total cost, and rationale of the agency for funding the infrastructure investment with funds made available under this Act, and name of the person to contact at the agency if there are concerns with the infrastructure investment.
(4) Detailed information on any subcontracts or subgrants awarded by the recipient to include the data elements required to comply with the Federal Funding Accountability and Transparency Act of 2006 (Public Law 109-282), allowing aggregate reporting on awards below $25,000 or to individuals, as prescribed by the Director of the Office of Management and Budget. OMB guidance for implementing and reporting ARRA activities can be found at http://www.whitehouse.gov/omb/recovery_default/.
D.Cooperative Agreement Terms and Conditions of Award
The
following special terms of award are in addition to, and not in lieu
of, otherwise applicable OMB administrative guidelines, HHS grant
administration regulations at 45 CFR Parts 74 and 92 (Part 92 is
applicable when State and local Governments are eligible to apply),
and other HHS, PHS, and ONC grant administration policies.
The
administrative and funding instrument used for this program will be
the cooperative agreement, an "assistance" mechanism, in
which substantial ONC programmatic involvement with the recipients is
anticipated during the performance of the activities. Under the
cooperative agreement, the ONC purpose is to support and stimulate
the recipients' activities by involvement in and otherwise working
jointly with the award recipients in a partnership role; it is not to
assume direction, prime responsibility, or a dominant role in the
activities. Consistent with this concept, the dominant role and prime
responsibility resides with the recipients for the project as a
whole, although specific tasks and activities may be shared among the
recipients and the ONC as defined below. To facilitate appropriate
involvement, during the period of this cooperative agreement, ONC and
the recipient will be in contact monthly and more frequently when
appropriate. Requests to modify or amend the cooperative agreement
may be made by ONC or the recipient at any time. Modifications and/or
amendments to the cooperative agreement shall be effective upon the
mutual agreement of both parties, except where ONC is authorized
under the Terms and Conditions of award, 45 CFR Part 74 or 92, or
other applicable regulation or statute to make unilateral
amendments.
1.Cooperative Agreement Roles and Responsibilities
Office of the National Coordinator for Health Information Technology
ONC will have substantial involvement in program awards, as outlined below:
Technical Assistance – This includes federal guidance on the evolution of HIE in accordance with meaningful use criteria to be established by the Secretary through the rulemaking process.
Over time ONC will also assist states in meeting the strategic goals of the state and overall program on a national level through ongoing support made available through the NHIN and other ONC funded programs.
Collaboration – To facilitate compliance with the terms of the cooperative agreement and to more effectively support recipients, ONC will actively coordinate with critical stakeholders, such as:
Medicaid and Medicare Administrators
State Designated Entities
State Government HIT Leads
Relevant Federal Agencies
Program Evaluation – As required by section 3013 of the HITECH Act, ONC will conduct a national level program evaluation and work with recipients to implement lessons learned to continuously improve this program and the nation-wide implementation of HIE.
Project Officers – ONC will assign specific Project Officers to each cooperative agreement award to support and monitor recipients throughout the period of performance.
Conference and Training Opportunities – ONC will host a minimum of two opportunities for training and/or networking, including, but not limited to, the State HIE Forum and Leadership Training.
Release of Funds Approval – ONC Project Officers will be responsible for requesting authorization for the release of funds for their assigned projects.
Monitoring – ONC Project Officers will monitor, on a regular basis, progress of each recipient. This monitoring may be by phone, document review, on-site visit, other meeting and by other appropriate means, such as reviewing program progress reports and Financial Status Reports (SF269). This monitoring will be to determine compliance with programmatic and financial requirements.
Recipients
Recipients and assigned points of contact retain the primary responsibility and dominant role for planning, directing and executing the proposed project as outlined in the terms and conditions of the cooperative agreement and with substantial ONC involvement. Responsibilities include:
Requirements – Recipients shall comply with all current and future requirements of the project, including those in their approved State Plans, guidance on the implementation of meaningful use, certification criteria and standards (including privacy and security) specified and approved by the Secretary of HHS
Participation in the State HIE Forum and Leadership Training.
Recipients are required to collaborate with the critical stakeholders listed in this Funding Opportunity Announcement and the ONC team, including the assigned Project Officer.
Recipients are required to collaborate with their Medicaid Directors to assist with monitoring and compliance of eligible meaningful use incentive recipients, to be established by the Secretary through the rulemaking process.
Recipients are required to collaborate with the Regional Centers to ensure that the provider connectivity supported by the Regional Centers is consistent with the State’s Plan for HIE.
Reporting – Recipients are required to comply with all reporting requirements outlined in this Funding Opportunity Announcement and the terms and conditions of the cooperative agreement to ensure the timely release of funds.
Program Evaluation – Recipients are required to cooperate with the ONC directed national program evaluation.
Dispute Resolution
Both ONC and the recipient are expected to work in a collegial fashion to minimize misunderstandings and disagreements. ONC will resolve disputes by using alternative dispute resolution (ADR) techniques. ADR often is effective in reducing the cost, delay, and contentiousness involved in appeals and other traditional ways of handling disputes. ONC will determine the specific technique to be employed on a case by case basis. ADR techniques include mediation, neutral evaluation, and other consensual methods. The National Coordinator for Health IT will make final determinations pertaining to cooperative agreements based on the output of these resolution methods.
2.Other Terms
These special terms and conditions of the award are in addition to and not in lieu of otherwise applicable OMB administrative guidelines, HHS grant administration regulations in 45 CFR, and other HHS and ONC policy statements.
Cooperative agreements are for a period of up to four years.
As meaningful use criteria to be established by the Secretary through the rulemaking process and other relevant guidance evolve, ONC will update ongoing program guidance. By accepting an award, recipients are required to abide by this guidance.
Drawdown of funding for this grant serves as official acceptance of this cooperative agreement. If you do not plan to accept the award, please send a letter of declination to the ONC Project Officer within 30 days of receipt of the Notice of Award.
Requests to modify or amend this cooperative agreement may be made at any time by ONC or the recipient, which shall be effective upon mutual agreement of both parties and if not agreed to will be subject to the dispute resolution practice below.
Recipients must comply with reporting requirements of the cooperative agreement.
Recipients must comply with the requirements of and cooperate with ONC in completing its responsibility to conduct a national evaluation.
Special conditions may be placed on cooperative agreements, based on the outcomes of negotiations with the applicants. These are binding on recipients. Among these conditions will be specific performance milestones with ties to funding availability. Available federal funds will be broken down into funding phases according to these milestones. During the course of the project period, recipients may drawdown funds as needed using the funds available to them for the phase they are in. At the achievement of the next milestone, such as the State Plan being approved by the National Coordinator, additional funding will become available for drawdown.
E.American Recovery and Reinvestment Act of 2009
1.HHS Standard Terms and Conditions
HHS award recipients must comply with all terms and conditions outlined in their award, including policy terms and conditions contained in applicable Department of Health and Human Services (HHS) Grant Policy Statements, and requirements imposed by program statutes and regulations and HHS grant administration regulations, as applicable, unless they conflict or are superseded by the following terms and conditions implementing the American Recovery and Reinvestment Act of 2009 (ARRA) requirements below. In addition to the standard terms and conditions of award, recipients receiving funds under Division A of ARRA must abide by the terms and conditions set out below. The terms and conditions below concerning civil rights obligations and disclosure of fraud and misconduct are reminders rather than new requirements, but the other requirements are new and are specifically imposed for awards funded under ARRA. Recipients are responsible for contacting their HHS grant/program managers/project officers for any needed clarifications.
Awards issued under this guidance are also subject to the requirements outlined in the HITECH Act, Section 3013 of ARRA.
2.Preference for Quick Start Activities
In using funds for this award for infrastructure investment, recipients shall give preference to activities that can be started and completed expeditiously, including a goal of using at least 50 percent of the funds for activities that can be initiated not later than 120 days after the date of the enactment of ARRA. Recipients shall also use funds in a manner that maximizes job creation and economic benefit. (ARRA Sec. 1602).
3.Limit on Funds
None of the funds appropriated or otherwise made available in ARRA may be used by any State or local government, or any private entity, for any casino or other gambling establishment, aquarium, zoo, golf course, or swimming pool. (ARRA Sec. 1604).
4.ARRA: One-Time Funding
Unless otherwise specified, ARRA funding to existent or new awardees should be considered one-time funding.
5.Civil Rights Obligations
While ARRA has not modified awardees’ civil rights obligations, which are referenced in the HHS’ Grants Policy Statement, these obligations remain a requirement of federal law. Recipients and sub-recipients of ARRA funds or other federal financial assistance must comply with Title VI of the Civil Rights Act of 1964 (prohibiting race, color, and national origin discrimination), Section 504 of the Rehabilitation Act of 1973 (prohibiting disability discrimination), Title IX of the Education Amendments of 1972 (prohibiting sex discrimination in education and training programs), and the Age Discrimination Act of 1975 (prohibiting age discrimination in the provision of services). For further information and technical assistance, please contact the HHS Office for Civil Rights at (202) 619-0403, OCRmail@hhs.gov, or http://www.hhs.gov/ocr/civilrights/.
6.Disclosure of Fraud or Misconduct
Each recipient or sub-recipient awarded funds made available under the ARRA shall promptly refer to the HHS Office of Inspector General any credible evidence that a principal, employee, agent, contractor, sub-recipient, subcontractor, or other person has submitted a false claim under the False Claims Act or has committed a criminal or civil violation of laws pertaining to fraud, conflict of interest, bribery, gratuity, or similar misconduct involving those funds. The HHS Office of Inspector General can be reached at http://www.oig.hhs.gov/fraud/hotline/.
7.Responsibilities for Informing Sub-recipients
Recipients agree to separately identify to each sub-recipient, and document at the time of sub-award and at the time of disbursement of funds, the federal award number, any special CFDA number assigned for ARRA purposes, and amount of ARRA funds.
Recovery Act Transactions listed in Schedule of Expenditures of Federal Awards and Recipient Responsibilities for Informing Sub-recipients
(a) To maximize the transparency and accountability of funds authorized under the American Recovery and Reinvestment Act of 2009 (Public Law 111-5) (ARRA) as required by Congress and in accordance with 45 CFR 74.21 and 92.20 "Uniform Administrative Requirements for Grants and Agreements", as applicable, and OMB A-102 Common Rules provisions, recipients agree to maintain records that identify adequately the source and application of ARRA funds.
(b) For recipients covered by the Single Audit Act Amendments of 1996 and OMB Circular A-133, "Audits of States, Local Governments, and Non-Profit Organizations," recipients agree to separately identify the expenditures for federal awards under ARRA on the Schedule of Expenditures of Federal Awards (SEFA) and the Data Collection Form (SF-SAC) required by OMB Circular A-133. This shall be accomplished by identifying expenditures for federal awards made under ARRA separately on the SEFA, and as separate rows under Item 9 of Part III on the SF-SAC by CFDA number, and inclusion of the prefix "ARRA-" in identifying the name of the federal program on the SEFA and as the first characters in Item 9d of Part III on the SF-SAC.
(c) Recipients agree to separately identify to each sub-recipient, and document at the time of sub-award and at the time of disbursement of funds, the federal award number, CFDA number, and amount of ARRA funds. When a recipient awards ARRA funds for an existing program, the information furnished to sub-recipients shall distinguish the sub-awards of incremental ARRA funds from regular sub-awards under the existing program.
(d) Recipients agree to require their sub-recipients to include on their SEFA information to specifically identify ARRA funding similar to the requirements for the recipient SEFA described above. This information is needed to allow the recipient to properly monitor sub-recipient expenditure of ARRA funds as well as oversight by the federal awarding agencies, Offices of Inspector General and the Government Accountability Office.
Recipient Reporting
Reporting and Registration Requirements under Section 1512 of the American Recovery and Reinvestment Act of 2009, Public Law 111-5
(a) This award requires the recipient to complete projects or activities which are funded under the American Recovery and Reinvestment Act of 2009 ("ARRA") and to report on use of ARRA funds provided through this award. Information from these reports will be made available to the public.
(b) The reports are due no later than ten calendar days after each calendar quarter in which the recipient receives the assistance award funded in whole or in part by ARRA.
(c) Recipients and their first-tier recipients must maintain current registrations in the Central Contractor Registration (www.ccr.gov) at all times during which they have active federal awards funded with ARRA funds. A Dun and Bradstreet Data Universal Numbering System (DUNS) Number (www.dnb.com) is one of the requirements for registration in the Central Contractor Registration.
(d) The recipient shall report the information described in section 1512(c) using the reporting instructions and data elements that will be provided online at http://www.FederalReporting.gov and ensure that any information that is pre-filled is corrected or updated as needed.
VII.Agency Contacts
Program Contact: Chris Muir Senior Program Analyst Office of the National Coordinator for Health Information Technology Department of Health and Human Services 200
Independence Avenue, S.W., Suite 729D Tel: (202) 205-0470 |
Grant Management Contact: Alexis Lynady Grant Management Specialist Assistant Secretary for Preparedness And Response Department of Health and Human Services 395 E Street, SW, Room 1075.42 Washington, D.C. 20201 Tel: (202)245-0976
|
This funding announcement is subject to restrictions on oral conversations during the period of time commencing with the submission of a formal application13 by an individual or entity and ending with the award of the competitive funds. Federal officials may not participate in oral communications initiated by any person or entity concerning a pending application for a Recovery Act competitive grant or other competitive form of Federal financial assistance, whether or not the initiating party is a federally registered lobbyist. This restriction applies unless:
(i) the communication is purely logistical;
(ii) the communication is made at a widely attended gathering;
(iii) the communication is to or from a Federal agency official and another Federal Government employee;
(iv) the communication is to or from a Federal agency official and an elected chief executive of a state, local or tribal government, or to or from a Federal agency official and the Presiding Officer or Majority Leader in each chamber of a state legislature; or
(v) the communication is initiated by the Federal agency official.
For additional information see http://www.whitehouse.gov/omb/assets/memoranda_fy2009/m09-24.pdf .
VIII.Appendices
A. State Grants to Promote Health Information Technology, authorized by Section 3013 of the PHSA as added by ARRA
B. Detailed Guidance for Strategic and Operational Plans
C. Required Content for Letter of Intent to Apply
D. Suggested Format for Letter from State Designating Official (Governor or Equivalent, for Territories)
E. Suggested Format for Letter of Support from Critical Stakeholders
F. Privacy and Security Resources
G. ARRA-Required Performance Measures
H. Public and Private Sector Models for Governance and Accountability
I. Instructions for completing the SF 424, Budget (SF 424A), Budget Narrative/Justification, and Other Required Forms
J. Budget Narrative/Justification, Page 1 – Sample Format with EXAMPLES
K. Budget Narrative/Justification –– Sample Template
L. Instructions for Completing the Project Summary/Abstract
M. Survey instructions on Ensuring Equal Opportunity for Applicants
N. Glossary of Terms
A.State Grants to Promote Health Information Technology, authorized by Section 3013 of the PHSA as added by ARRA
“SEC. 3013. STATE GRANTS TO PROMOTE HEALTH INFORMATION TECHNOLOGY.
‘‘(a) IN GENERAL.—The Secretary, acting through the National Coordinator, shall establish a program in accordance with this section to facilitate and expand the electronic movement and use of health information among organizations according to nationally recognized standards.
‘‘(b) PLANNING GRANTS.—The Secretary may award a grant to a State or qualified State-designated entity (as described in subsection (f)) that submits an application to the Secretary at such time, in such manner, and containing such information as the Secretary may specify, for the purpose of planning activities described in subsection (d).
‘‘(c) IMPLEMENTATION GRANTS.—The Secretary may award a grant to a State or qualified State designated entity that—
‘‘(1) has submitted, and the Secretary has approved, a plan described in subsection (e) (regardless of whether such plan was prepared using amounts awarded under subsection (b); and
‘‘(2) submits an application at such time, in such manner, and containing such information as the Secretary may specify.
‘‘(d) USE OF FUNDS.—Amounts received under a grant under subsection (c) shall be used to conduct activities to facilitate and expand the electronic movement and use of health information among organizations according to nationally recognized standards through activities that include—
‘‘(1) enhancing broad and varied participation in the authorized and secure nationwide electronic use and exchange of health information;
‘‘(2) identifying State or local resources available towards a nationwide effort to promote health information technology;
‘‘(3) complementing other Federal grants, programs, and efforts towards the promotion of health information technology;
‘‘(4) providing technical assistance for the development and dissemination of solutions to barriers to the exchange of electronic health information;
‘‘(5) promoting effective strategies to adopt and utilize health information technology in medically underserved
communities;
‘‘(6) assisting patients in utilizing health information technology;
‘‘(7) encouraging clinicians to work with Health Information Technology Regional Extension Centers as described in section 3012, to the extent they are available and valuable;
‘‘(8) supporting public health agencies’ authorized use of and access to electronic health information;
‘‘(9) promoting the use of electronic health records for quality improvement including through quality measures
reporting; and
‘‘(10) such other activities as the Secretary may specify.
‘‘(e) PLAN.—
‘‘(1) IN GENERAL.—A plan described in this subsection is a plan that describes the activities to be carried out by a State or by the qualified State-designated entity within such State to facilitate and expand the electronic movement and use of health information among organizations according to nationally recognized standards and implementation specifications.
‘‘(2) REQUIRED ELEMENTS.—A plan described in paragraph (1) shall—
‘‘(A) be pursued in the public interest;
‘‘(B) be consistent with the strategic plan developed by the National Coordinator, (and, as available) under section 3001;
‘‘(C) include a description of the ways the State or qualified State-designated entity will carry out the activities described in subsection (b); and
‘‘(D) contain such elements as the Secretary may require.
‘‘(f)
QUALIFIED STATE-DESIGNATED ENTITY.—For purposes of this
section, to be a qualified State-designated entity, with respect to a
State, an entity shall—
‘‘(1) be designated by the State as eligible to receive awards under this section;
‘‘(2) be a not-for-profit entity with broad stakeholder representation on its governing board;
‘‘(3) demonstrate that one of its principal goals is to use information technology to improve health care quality and efficiency through the authorized and secure electronic exchange and use of health information;
‘‘(4) adopt nondiscrimination and conflict of interest policies that demonstrate a commitment to open, fair, and nondiscriminatory participation by stakeholders; and
‘‘(5) conform to such other requirements as the Secretary may establish.
‘‘(g) REQUIRED CONSULTATION.—In carrying out activities described in subsections (b) and (c), a State or qualified State designated entity shall consult with and consider the recommendations of—
‘‘(1) health care providers (including providers that provide services to low income and underserved populations);
‘‘(2) health plans;
‘‘(3) patient or consumer organizations that represent the population to be served;
‘‘(4) health information technology vendors;
‘‘(5) health care purchasers and employers;
‘‘(6) public health agencies;
‘‘(7) health professions schools, universities and colleges;
‘‘(8) clinical researchers;
‘‘(9) other users of health information technology such as the support and clerical staff of providers and others involved in the care and care coordination of patients; and
‘‘(10) such other entities, as may be determined appropriate by the Secretary.
‘‘(h) CONTINUOUS IMPROVEMENT.—The Secretary shall annually evaluate the activities conducted under this section and shall, in awarding grants under this section, implement the lessons learned from such evaluation in a manner so that awards made subsequent to each such evaluation are made in a manner that, in the determination of the Secretary, will lead towards the greatest improvement in quality of care, decrease in costs, and the most effective
authorized and secure electronic exchange of health information.
‘‘(i) REQUIRED MATCH.—
‘‘(1) IN GENERAL.—For a fiscal year (beginning with fiscal year 2011), the Secretary may not make a grant under this section to a State unless the State agrees to make available non-Federal contributions (which may include in-kind contributions) toward the costs of a grant awarded under subsection (c) in an amount equal to—
‘‘(A) for fiscal year 2011, not less than $1 for each $10 of Federal funds provided under the grant;
‘‘(B) for fiscal year 2012, not less than $1 for each $7 of Federal funds provided under the grant; and
‘‘(C) for fiscal year 2013 and each subsequent fiscal year, not less than $1 for each $3 of Federal funds provided under the grant.
‘‘(2) AUTHORITY TO REQUIRE STATE MATCH FOR FISCAL YEARS BEFORE FISCAL YEAR 2011.—For any fiscal year during the grant program under this section before fiscal year 2011, the Secretary may determine the extent to which there shall be required a non-Federal contribution from a State receiving a grant under this section.”
B.Detailed Guidance for Strategic and Operational Plans
1.Detailed Guidance for the Strategic Plan
The strategic planning process includes the development of the initial Strategic Plan and ongoing updates. There are distinct and/or concurrent planning activities for each domain that need to be coordinated and planned. The Strategic Plan may address the evolution of capabilities supporting HIE, as well as progress in the five domains of HIE activity, the role of partners and stakeholders, and high-level project descriptions for planning, implementation, and evaluation.
The following criteria in General Topic Guidance and Domain Requirements must be included in the Strategic and Operational plans unless noted as otherwise.
a)General Topic Guidance
Environmental Scan – The Strategic Plan must include an environmental scan of HIE readiness which may include broad adoption of HIT but must include HIE adoption across health care providers within the state and potentially external to the state, as relevant. The environmental scan must include an assessment of current HIE capacities that could be expanded or leveraged, HIT resources that could be used, the relevant collaborative opportunities that already exist, the human capital that is available and other information that indicates the readiness of HIE implementation statewide.
HIE Development and Adoption – The Strategic plan must address vision, goals, objectives and strategies associated with HIE capacity development and use among all health care providers in the state, to include meeting HIE meaningful use criteria to be established by the Secretary through the rulemaking process. The Strategic Plan must also address continuous improvement in realizing appropriate and secure HIE across health care providers for care coordination and improvements to quality and efficiency of health care. Strategic Plans should also address HIE between health care providers, public health, and those offering services for patient engagement and data access.
HIT Adoption (encouraged but not required)–
HIT adoption may also be included in the Strategic Plan. Although it is beyond the scope of this program to fund HIT adoption initiatives described in a State Strategic Plan, it does not preclude other HITECH ACT programs or state funded initiatives to advance HIT adoption in a state.
While many states have already addressed HIT adoption in their existing Health IT State Plans, it is not a requirement. However, the inclusion of Health IT adoption in the Strategic Plan is valuable and provides for a more comprehensive approach for planning how to achieve connectivity across the state.
Medicaid Coordination – The Strategic Plan must describe the interdependencies and integration of efforts between the state’s Medicaid HIT Plan and the statewide HIE development efforts. The description should include the state’s HIE related requirements for meaningful use to be established by the Secretary through the rulemaking process and the mechanisms in which the state will measure provider participation in HIE.
Coordination of Medicare and Federally Funded, State Based Programs – Strategic Plan shall describe the coordination activities with Medicare and relevant federally-funded, state programs (see program guidance). These programs include:
Epidemiology and Laboratory Capacity Cooperative Agreement Program (CDC)
Assistance for Integrating the Long-Term Care Population into State Grants to Promote Health IT
Implementation (CMS/ASPE)
HIV Care Grant Program Part B States/Territories Formula and Supplemental Awards/AIDS Drug Assistance Program Formula and Supplemental Awards (HRSA)
Maternal and Child Health State Systems Development Initiative programs (HRSA)
State Offices of Rural Health Policy (HRSA)
State Offices of Primary Care (HRSA)
State Mental Health Data Infrastructure Grants for Quality Improvement (SAMHSA)
State Medicaid/CHIP Programs
IHS and tribal activity
Emergency Medical Services for Children Program (HRSA)
Participation with federal care delivery organizations (encouraged but not required)– When applicable, the Strategic Plan should include a description of the extent to which the various federal care delivery organizations, including but not limited to the VA, DoD, and IHS, will be participating in state activities related to HIE.
Coordination of Other ARRA Programs – Because other ARRA funding will be available to the state that can help advance HIE, the Strategic Plan must describe, when applicable, coordination mechanisms with other relevant ARRA programs including Regional Centers, workforce development initiatives, and broadband mapping and access. As these programs are developed, ONC will provide program guidance to facilitate state specific coordination across Regional Centers, workforce development and broadband programs. For planning purposes, applicants should specify how entities or collaboratives planning to be Regional Centers will provide technical assistance to health care providers in their states, how trained professionals from workforce development programs will be utilized to support statewide HIE, and how plans to expand access to broadband will inform State Strategic and Operational Plans overtime. This program coordination will be the subject of future guidance, and plans may need to be modified as other programs are clarified.
b)Domain Requirements
Governance
Collaborative Governance Model – The Strategic Plan must describe the multi-disciplinary, multi-stakeholder governance entity including a description of the membership, decision-making authority, and governance model. States are encouraged to consider how their state governance models will align with emerging nationwide HIE governance.
State Government HIT Coordinator – The Strategic Plan shall identify the state Government HIT Coordinator. The plan shall also describe how the state coordinator will interact with the federally funded state health programs and also the HIE activities within the state.
Accountability and Transparency – To ensure that HIE is pursued in the public’s interest, the Strategic Plan shall address how the state is going to address HIE accountability and transparency.
Finance
Sustainability – In order to ensure the financial sustainability of the project beyond the ARRA funding, the Strategic Plan shall include a business plan that enables for the financial sustainability, by the end of the project period of HIE governance and operations.
Technical Infrastructure
Interoperability - The plan must indicate whether the HIE services will include participation in the NHIN. The plan shall include the appropriate HHS adopted standards and certifications for health information exchange, especially planning and accounting for meaningful use criteria to be established by the Secretary through the rulemaking process .
Technical Architecture/Approach (encouraged but not required)– Because the state or SDE may or may not implement HIE, the Strategic Plan may include an outline of the data and technical architectures and describe the approach to be used, including the HIE services to be offered as appropriate for the state’s HIE capacity development.
Business and Technical Operations
Implementation – To address how the state plans will develop HIE capacity, the Strategic Plan must include a strategy that specifies how the state intends to meet meaningful use HIE requirements established by the Secretary, leverage existing state and regional HIE capacity and leverage statewide shared services and directories. The implementation strategy described in the Strategic Plan shall describe the incremental approach for HIE services to reach all geographies and providers across the state. The implementation strategy shall identify if and when the state HIE infrastructure will participate in the NHIN.
Legal/policy
Privacy and Security– The Strategic Plan shall address privacy and security issues related to health information exchange within the state, and between states. The plan shall give special attention to federal and state laws and regulations and adherence to the privacy principles articulated in the HHS Privacy and Security Framework, and any related guidance.
State Laws – The Strategic Plan shall address any plans to analyze and/or modify state laws, as well as communications and negotiations with other states to enable exchange.
Policies and Procedures – The Strategic Plan shall also address the development of policies and procedures necessary to enable and foster information exchange within the state and interstate.
Trust Agreements –The Strategic Plan shall discuss the use of existing or the development of new trust agreements among parties to the information exchange that enable the secure flow of information. Trust agreements include but are not limited to data sharing agreements, data use agreements and reciprocal support agreements.
Oversight of Information Exchange and Enforcement - The Strategic Plan shall address how the state will address issues of noncompliance with federal and state laws and policies applicable to HIE.
2.Detailed Guidance for the Operational Plan
Prior to entering into funded implementation activities, a state must submit and receive approval of the Operational Plan. The Operational Plan shall include details on how the Strategic Plan will be carried forward and executed to enable statewide HIE. It must also include a project schedule describing the tasks and sub-tasks that need to be completed in order to enable the statewide HIE. The implementation description shall identify issues, risks, and interdependencies within the overall project. In addition, the Operational Plan must include the following general topics and domains. The requirements for the initial Operational Plan are outlined below.
a)General Topic Requirements
Coordinate with ARRA Programs – The Operational Plan must describe specific points of coordination and interdependencies with other relevant ARRA programs including Regional Centers, workforce development initiatives, and broadband mapping and access. As these programs are developed, ONC will provide program guidance to facilitate state specific coordination across Regional Centers, workforce development and broadband programs. For planning purposes, applicants concurrently applying as HIE recipients and Regional Center recipients should specify how they will provide technical assistance to health care providers in their states with estimates of geographic and provider coverage. In addition, project resource planning should take into account how and when trained professionals from workforce development programs will be utilized to support statewide HIE, and how and when broadband will be available to health care providers across the state according to the availability of up to date broadband maps and funded efforts to expand access.
Coordinate with Other States – In order to share lessons learned and encourage scalable solutions between states, the Operational Plan shall describe multi-state coordination activities including the sharing of plans between states.
b)Domain Requirements
Governance
Governance and Policy Structures – The Operational Plan must describe the ongoing development of the governance and policy structures.
Finance
Cost Estimates and Staffing Plans – The Operational Plan must provide a detailed cost estimate for the implementation of the Strategic Plan for the time period covered by the Operational Plan. It must also include a detailed schedule describing the tasks and sub-tasks that need to be completed in order to enable statewide HIE along with resources, dependencies, and specific timeframes. The implementation description shall specify proposed resolution and mitigation methods for identified issues and risks within the overall project. Additionally, recipients shall provide staffing plans including project managers and other key roles required to ensure the project’s success.
Controls and Reporting – The Operational Plan must describe activities to implement financial policies, procedures and controls to maintain compliance with generally accepted accounting principles (GAAP) and all relevant OMB circulars. The organization will serve as a single point of contact to submit progress and spending reports periodically to ONC.
Technical Infrastructure
Standards and Certifications –The Operational Plan shall describe efforts to become consistent with HHS adopted interoperability standards and any certification requirements, for projects that are just starting; demonstrated compliance, or plans toward becoming consistent with HHS adopted interoperability standards and certifications if applicable, for those projects that are already implemented or under implementation.
Technical Architecture – The Operational Plan must describe how the technical architecture will accommodate the requirements to ensure statewide availability of HIE among healthcare providers, public health and those offering service for patient engagement and data access. The technical architecture must include plans for the protection of health data. This needs to reflect the business and clinical requirements determined via the multi-stakeholder planning process. If a state plans to exchange information with federal health care providers including but not limited to VA, DoD, IHS, their plans must specify how the architecture will align with NHIN core services and specifications.
Technology Deployment – The Operational Plan must describe the technical solutions that will be used to develop HIE capacity within the state and particularly the solutions that will enable meaningful use criteria established by the Secretary for 2011, and indicate efforts for nationwide health information exchange. If a state plans to participate in the Nationwide Health Information Network (NHIN), their plans must specify how they will be complaint with HHS adopted standards and implementation specifications. (For up-to-date publicly available information on meaningful use, see: http://healthit.hhs.gov/meaningfuluse).
Business and Technical operations
Current HIE Capacities – The Operational Plan must describe how the state will leverage current HIE capacities, if applicable, such as current operational health information organizations (HIOs), including those providing services to areas in multiple states.
State-Level Shared Services and Repositories – The Operational Plan must address whether the state will leverage state-level shared services and repositories including how HIOs and other data exchange mechanisms can leverage existing services and data repositories, both public or private. Shared services for states to consider include (but are not limited to): Security Service, Patient Locator Service, Data/Document Locator Service, and Terminology Service. These technical services may be developed over time and according to standards and certification criteria adopted by HHS in effort to develop capacity for nationwide HIE.
Standard operating procedures for HIE (encouraged but not required)– The Operational Plan should include an explanation of how standard operating procedures and processes for HIE services will be developed and implemented.
Legal/policy
Establish Requirements – The Operational Plan shall describe how statewide health information exchange will comply with all applicable federal and state legal and policy requirements. This plan needs to include developing, evolving, and implementing the policy requirements to enable appropriate and secure health information exchange through the mechanisms of exchange consistent with the state Strategic Plan. The Operational Plan should specify the interdependence with the governance and oversight mechanisms to ensure compliance with these policies.
Privacy and Security Harmonization – The Operational Plan must describe plans for privacy and security harmonization and compliance statewide and also coordination activities to establish consistency on an interstate basis.
Federal Requirements – To the extent that states anticipate exchanging health information with federal care delivery organizations, such as the VA, DoD, Indian Health Service, etc. the Operational Plan must consider the various federal requirements for the utilization and protection of health data will be accomplished.
C.Required Content for Letter of Intent to Apply
Prospective applicants must submit a Letter of Intent that includes the following information. (For multi-state applications, only one letter of intent should be submitted. This letter should be submitted by the state or SDE that will act as the applicant on behalf of all states involved in the proposed project.):
Descriptive title of proposed project.
Indication of whether a State Plan already exists or will be developed during the life of this cooperative agreement.
Will the application submitted be for more than one state/territory? If so, which states/territories will be included?
Name, address, and telephone number of the primary Point of Contact.
Names of other key personnel.
Participating stakeholders.
Does the applicant for this program intend to apply to be a Regional Center as well?
Number and title of this funding opportunity.
A brief description of your state’s progress in each of the domain areas below, as well as, a brief description of the state’s intentions to leverage existing regional efforts to advance health information exchange.The total amount of expenditures to develop HIE capacity based on funded activities in the following domains:
Legal and policy HIE capacity: Types of activities include but are not limited to expenses incurred to create: data use agreements, business associate agreements, vendor contracts, privacy policies and procedures, governance documents, employee policies and procedures, and legal opinions.
Governance capacity: Types of activities include but are not limited to expenses incurred to: convene health care stakeholders, create plans for statewide coverage of HIE services; provide oversight and accountability of health information exchange activities.
Business and Technical Operations capacity: Types of activities include but are not limited to expenses incurred to: develop and operate the technical services needed for health information exchange on a national, state and regional level, support activities including procurement, functionality development, project management, help desk, systems maintenance, change control, program evaluation, reporting and other related activities, legal and policy documents that support HIE enabled meaningful use criteria to be established by the Secretary through the rulemaking process.
Technical infrastructure capacity: Types of activities include but are not limited to expenses incurred to: developed the architecture, hardware, software, applications, network configurations and other technological aspects that physically enable health information exchange in a secure and appropriate manner that also meets overarching goals for a high performance health care system.
Finance capacity: Types of activities include but are not limited to expenses incurred to: develop and manage finance policies procedure and controls, sustainability plans, pricing strategies, market research, public and private financing strategies, financial reporting, business planning, and audits.
A brief description of your state’s progress in each of the areas above, as well as, a brief description of the state’s intentions to leverage existing regional efforts to advance health information exchange.
Explanation of how the proposed project will be in the public interest.
A letter of intent is not binding, and does not enter into the review of a subsequent application, the information that it contains allows ONC staff members to estimate the potential review workload and plan the review.
The letter of intent should be no longer than 5 pages and can be sent by the date listed in the Important Dates table above (Opportunity Overview).
The letter of intent shall be sent to at the following address:
David Blumenthal MD, MPP
National Coordinator for Health Information Technology
Department of Health and Human Services
200
Independence Avenue, S.W.
Washington, DC 20201
Tel: (202) 690-7151
StateHIEgrants@hhs.gov
D.Suggested Format for Letter from State Designating Official (Governor or Equivalent, for Territories)
Designating Official is the Governor. For territories and the District of Columbia, it is the Equivalent Official (i.e. Mayor). For multi-state applications, a letter from the Governor (or equivalent) designating the partnering state or SDE must be received on behalf of each state participating in the proposed project.
David Blumenthal MD, MPP
National Coordinator for Health Information Technology
Department of Health and Human Services
200
Independence Avenue, S.W.
Washington, DC 20201
Date
Dear Dr. Blumenthal,
The official (State Agency/State Designated Entity) for the State Grants to Promote Health Information Technology Program, for the State/Commonwealth/Territory of ______ is:
Name
Title
Agency
Division (if applicable)
State
Address
Phone
Fax Number
Governor’s (or equivalent) Signature
E.Suggested Format for Letter of Support from Critical Stakeholders
David Blumenthal MD, MPP
National Coordinator for Health Information Technology
Department of Health and Human Services
200
Independence Avenue, S.W.
Washington, DC 20201
Date
Dear
Dr. Blumenthal,
(Name of organization/group submitting the letter) is very interested in addressing (insert the issue being addressed by the grant application.) and (State why the issue is of concern.)
(State knowledge of proposal, knowledge of agency submitting proposal, and encouragement of funding entity to provide resources to address issue identified above.)
(State that the need to address the issue is significant and how other resources to address the need are insufficient to address or impact the need.)
(Specifically state how your organization will support this project – through assistance with meeting matching requirements, board/commission participation, advocacy)
(State that the proposing organization would coordinate with appropriate partners to ensure efficient and effective use of grant funds.)
(Conclude with general statement of confidence in and support for the organization seeking assistance, based on past experience with the applicant entity, reputation for effectiveness)
(Provide the following information for the point of contact in the supporting organization.)
Name
Title
Agency
Division (if applicable)
State
Address
Phone
Fax Number
F.Privacy and Security Resources
American Reinvestment and ARRA References
ARRA Section D – Privacy describes improved privacy provisions and security provisions related to:
Sec. 13402 - notification in the case of breach
Sec. 13404 – application of privacy provisions and penalties to business associates of covered entities
Sec. 13405 – restrictions on certain disclosures and sales of health information; accounting of certain protected health information disclosures; access to certain information in electronic format
Sec. 13406 – conditions on certain contacts as part of health care operations
Sec. 13407 – temporary breach notification requirement for vendors of personal health records and other non-HIPAA covered entities
Sec. 13408 – business associate contracts required for certain entities
This list is provided to highlight examples of the ARRA privacy and security requirements. It is not intended to be comprehensive, nor definitive program guidance to recipients regarding the ARRA requirements for privacy and security. To read a full version of ARRA, click here.
Privacy Act of 1974
45.C.F.R. Part 5b A link to the full Privacy Act can be found at: http://www.hhs.gov/foia/privacy/index.html
HIPAA Security Rule
45 CFR Parts 160, 162, and 164.
A link to the HIPAA Security Rule can be found http://www.hhs.gov/ocr/privacy/hipaa/administrative/privacyrule/adminsimpregtext.pdf.
HIPAA Privacy Rule
45 CFR Part 160 and Subparts A and E of Part 164. For more details: http://www.hhs.gov/ocr/privacy/hipaa/administrative/privacyrule/adminsimpregtext.pdf
Federal Information Security Management Act, 2002
45 CFR Parts 160, 162, and 164. A link to the full Act can be found at:
http://aspe.hhs.gov/datacncl/Privacy/titleV.pdf
Confidentiality of Alcohol and Drug Abuse Patient Records
45 CFR Part 2
For more details: http://www.hipaa.samhsa.gov
The HHS Privacy and Security Framework Principles
Individual Access - Individuals should be provided with a simple and timely means to access and obtain their individually identifiable health information in a readable form and format.
Correction- Individuals should be provided with a timely means to dispute the accuracy or integrity of their individually identifiable health information, and to have erroneous information corrected or to have a dispute documented if their requests are denied.
Openness and Transparency - There should be openness and transparency about policies, procedures, and technologies that directly affect individuals and/or their individually identifiable health information.
Individual Choice - Individuals should be provided a reasonable opportunity and capability to make informed decisions about the collection, use, and disclosure of their individually identifiable health information.
Collection, Use and Disclosure Limitation - Individually identifiable health information should be collected, used, and/or disclosed only to the extent necessary to accomplish a specified purpose(s) and never to discriminate inappropriately.
Data Quality and Integrity - Persons and entities should take reasonable steps to ensure that individually identifiable health information is complete, accurate, and up-to-date to the extent necessary for the person’s or entity’s intended purposes and has not been altered or destroyed in an unauthorized manner.
Safeguards - Individually identifiable health information should be protected with reasonable administrative, technical, and physical safeguards to ensure its confidentiality, integrity, and availability and to prevent unauthorized or inappropriate access, use, or disclosure.
Accountability - These principles should be implemented, and adherence assured, through appropriate monitoring and other means and methods should be in place to report and mitigate non-adherence and breaches.
For more information, please visit healthit.hhs.gov and click on the Privacy and Security link for the Framework and its Principles, or click here.
G.ARRA-Required Performance Measures
To assist in fulfilling the accountability objectives of the Recovery Act, as well as the Department’s responsibilities under the Government Performance and Results Act of 1993 (GPRA), Public Law 103-62, applicants who receive funding under this program must provide data that measure the results of their work. Additionally, applicants must discuss their data collection methods in the application. The following are required measures for awards made under the Recovery Act:
-
Objective
Performance Measures
Data the recipient provides for 3-month reporting period
Description
(Plain language explanation of what exactly is being provided)
Recovery Act: Preserving jobs
Number of jobs saved (by type) due to Recovery Act funding.
a) How many jobs were prevented from being eliminated with the Recovery Act funding during this reporting period?
b) How many jobs that were eliminated within the last 12 months were reinstated with Recovery Act funding?
An unduplicated number of jobs that would have been eliminated if not for the Recovery Act funding during the three-month quarter. Report this data for each position only once during the project period. A job can include full time, part time, contractual, or other employment relationship.
Recovery Act: Creating jobs
Number of jobs created (by type) due to Recovery Act funding.
How many jobs were created with Recovery Act funding this reporting period?
An unduplicated number of jobs created due to Recovery Act funding during the three month quarter. Report this data for each position only once during the award. A job can include full time, part time, contractual, or other employment relationship.
H.Public and Private Sector Models for Governance and Accountability
According to the National Governors Association (NGA) report on Public Governance Models for a Sustainable Health Information Exchange Industry, there are three types of legal structures that are utilized in a public sector model including the public authority model, the non-profit government controlled model, or the state agency model. The public authority model is part of the state government and subject to requirements of due process, open meetings, and public records. The government controlled non-profit corporation model is typically created by statue and includes a majority interest of state government board members on a separate non-profit board. Lastly, with the state agency model the HIE planning and implementation becomes the responsibility of an existing state agency. As for accountability, public sector controlled models typically leverage contract mechanisms to provide public accountability for privacy, security, fiscal integrity, system interoperability, and auditing of system access. Additional governmental accountability is provided through legislative reporting processes.
The private non-profit corporations usually utilize a governance structure whereby directors and officers are responsible for working with management to set strategy and adopt policies for HIE operation. The bylaws of any private non-profit corporation spell out the details of board composition, voting rights, board member terms and subcommittee composition. For accountability, private non-profit boards execute non-discrimination and conflict of interest policies that demonstrate a commitment to open, fair, and nondiscriminatory board activities. In addition, to ensure trust and buy-in, organization activities are usually open to the public and described in an annual activities report.
I.Instructions for completing the SF 424, Budget (SF 424A), Budget Narrative/Justification, and Other Required Forms
This section provides step-by-step instructions for completing the four (4) standard federal forms required as part of your grant application, including special instructions for completing Standard Budget Forms 424 and 424A. Standard Forms 424 and 424A are used for a wide variety of federal grant programs, and federal agencies have the discretion to require some or all of the information on these forms. Accordingly, please use the instructions below in lieu of the standard instructions attached to SF 424 and 424A to complete these forms. |
a. Standard Form 424
1. Type of Submission: (Required): Select one type of submission in accordance with agency instructions.
• Preapplication • Application • Changed/Corrected Application – If requested, check if this submission is to change or correct a previously submitted application.
2. Type of Application: (Required) Select one type of application in accordance with agency instructions.
• New . • Continuation • Revision
3. Date Received: Leave this field blank.
4. Applicant Identifier: Leave this field blank.
5a Federal Entity Identifier: Leave this field blank.
5b. Federal Award Identifier: For new applications leave blank. For a continuation or revision to an existing award, enter the previously assigned federal award (grant) number.
6. Date Received by State: Leave this field blank.
7. State Application Identifier: Leave this field blank.
8. Applicant Information: Enter the following in accordance with agency instructions:
a. Legal Name: (Required): Enter the name that the organization has registered with the Central Contractor Registry. Information on registering with CCR may be obtained by visiting the Grants.gov website.
b. Employer/Taxpayer Number (EIN/TIN): (Required): Enter the Employer or Taxpayer Identification Number (EIN or TIN) as assigned by the Internal Revenue Service.
c. Organizational DUNS: (Required) Enter the organization’s DUNS or DUNS+4 number received from Dun and Bradstreet. Information on obtaining a DUNS number may be obtained by visiting the Grants.gov website.
d. Address: (Required) Enter the complete address including the county.
e. Organizational Unit: Enter the name of the primary organizational unit (and department or division, if applicable) that will undertake the project.
f. Name and contact information of person to be contacted on matters involving this application: Enter the name (First and last name required), organizational affiliation (if affiliated with an organization other than the applicant organization), telephone number (Required), fax number, and email address (Required) of the person to contact on matters related to this application.
9. Type of Applicant: (Required) Select the applicant organization “type” from the following drop down list.
A. State Government B. County Government C. City or Township Government D. Special District Government E. Regional Organization F. U.S. Territory or Possession G. Independent School District H. Public/State Controlled Institution of Higher Education I. Indian/Native American Tribal Government (Federally Recognized) J. Indian/Native American Tribal Government (Other than Federally Recognized) K. Indian/Native American Tribally Designated Organization L. Public/Indian Housing Authority M. Nonprofit with 501C3 IRS Status (Other than Institution of Higher Education) N. Nonprofit without 501C3 IRS Status (Other than Institution of Higher Education) O. Private Institution of Higher Education P. Individual Q. For-Profit Organization (Other than Small Business) R. Small Business S. Hispanic-serving Institution T. Historically Black Colleges and Universities (HBCUs) U. Tribally Controlled Colleges and Universities (TCCUs) V. Alaska Native and Native Hawaiian Serving Institutions W. Non-domestic (non-US) Entity X. Other (specify)
10. Name Of Federal Agency: (Required) Enter U.S. Assistant Secretary for Preparedness and Response
11. Catalog Of Federal Domestic Assistance Number/Title: The CFDA number can be found on page one of the Program Announcement.
12. Funding Opportunity Number/Title: (Required) The Funding Opportunity Number and title of the opportunity can be found on page one of the Program Announcement.
13. Competition Identification Number/Title: Leave this field blank.
14. Areas Affected By Project: List the largest political entity affected (cities, counties, state).
15. Descriptive Title of Applicant’s Project: (Required) Enter a brief descriptive title of the project.
16. Congressional Districts Of: (Required) 16a. Enter the applicant’s Congressional District, and 16b. Enter all district(s) affected by the program or project. Enter in the format: 2 characters State Abbreviation – 3 characters District Number, e.g., CA-005 for California 5th district, CA-012 for California 12th district, NC-103 for North Carolina’s 103rd district. • If all congressional districts in a state are affected, enter “all” for the district number, e.g., MD-all for all congressional districts in Maryland. • If nationwide, i.e. all districts within all states are affected, enter US-all.
17. Proposed Project Start and End Dates: (Required) Enter the proposed start date and final end date of the project. Therefore, if you are applying for a multi-year grant, such as a 3 year grant project, the final project end date will be 3 years after the proposed start date.
18. Estimated Funding: (Required) Enter the amount requested or to be contributed during the first funding/budget period by each contributor. Value of in-kind contributions should be included on appropriate lines, as applicable. If the action will result in a dollar change to an existing award, indicate only the amount of the change. For decreases, enclose the amounts in parentheses.
NOTE: Applicants should review matching principles contained in Subpart C of 45 CFR Part 74 or 45 CFR Part 92 before completing Item 18 and the Budget Information Sections A, B and C noted below.
All budget information entered under item 18 should cover the upcoming budget period. For sub-item 18a, enter the federal funds being requested. Sub-items 18b-18e is considered matching funds. The dollar amounts entered in sub-items 18b-18f must total at least 1/3rd of the amount of federal funds being requested (the amount in 18a). For a full explanation of ONC’s match requirements, see the information in the box below. For sub-item 18f, enter only the amount, if any, which is going to be used as part of the required match.
There are two types of match: 1) non-federal cash and 2) non-federal in-kind. In general, costs borne by the applicant and cash contributions of any and all third parties involved in the project, including sub-grantees, contractors and consultants, are considered matching funds. Generally, most contributions from sub-contractors or sub-grantees (third parties) will be non-federal in-kind matching funds. Volunteered time and use of facilities to hold meetings or conduct project activities may be considered in-kind (third party) donations. Examples of non-federal cash match include budgetary funds provided from the applicant agency’s budget for costs associated with the project.
NOTE: Indirect charges may only be requested if: (1) the applicant has a current indirect cost rate agreement approved by the Department of Health and Human Services or another federal agency; or (2) the applicant is a state or local government agency. State governments should enter the amount of indirect costs determined in accordance with DHHS requirements. If indirect costs are to be included in the application, a copy of the approved indirect cost agreement must be included with the application. Further, if any sub-contractors or sub-grantees are requesting indirect costs, copies of their indirect cost agreements must also be included with the application.
ONC’s Match
Requirement Under
this program, the applicant’s match requirement is $1 for
every $10 Federal dollars for the first year of the program (FY2011)
In other words, for every ten (10) dollars received in Federal
funding, the applicant must contribute at least one (1) dollar in
non-Federal resources toward the project’s total cost. This
“ten-to-one” ratio is reflected in the following formula
which you can use to calculate your minimum required match:
Federal Funds
Requested 10
= Minimum Match
Requirement For
example, if you request $100,000 in Federal funds, then your minimum
match requirement is $100,000/10 or $10,000. In this example the
project’s total cost would be $110,000.
If
the required non-Federal share is not met by a funded project, ONC
will disallow any unmatched Federal dollars.
19. Is Application Subject to Review by State Under Executive Order 12372 Process? Check c. Program is not covered by E.O. 12372.
20. Is the Applicant Delinquent on any Federal Debt? (Required) This question applies to the applicant organization, not the person who signs as the authorized representative. If yes, include an explanation on the continuation sheet.
21. Authorized Representative: (Required) To be signed and dated by the authorized representative of the applicant organization. Enter the name (First and last name required) title (Required), telephone number (Required), fax number, and email address (Required) of the person authorized to sign for the applicant. A copy of the governing body’s authorization for you to sign this application as the official representative must be on file in the applicant’s office. (Certain federal agencies may require that this authorization be submitted as part of the application.)
b. Standard Form 424A
NOTE: Standard Form 424A is designed to accommodate applications for multiple grant programs; thus, for purposes of this program, many of the budget item columns and rows are not applicable. You should only consider and respond to the budget items for which guidance is provided below. Unless otherwise indicated, the SF 424A should reflect a one year budget. |
Section A ‑ Budget Summary
Line 5: Leave columns (c) and (d) blank. Enter TOTAL federal costs in column (e) and total non‑federal costs (including third party in‑kind contributions and any program income to be used as part of the grantee match) in column (f). Enter the sum of columns (e) and (f) in column (g).
Section B ‑ Budget Categories
Column 3: Enter the breakdown of how you plan to use the federal funds being requested by object class category (see instructions for each object class category below).
Column 4: Enter the breakdown of how you plan to use the non-federal share by object class category.
Column 5: Enter the total funds required for the project (sum of Columns 3 and 4) by object class category.
Separate Budget Narrative/Justification Requirement
You must submit a separate Budget Narrative/Justification as part of your application. When more than 33% of a project’s total budget falls under contractual, detailed Budget Narratives/Justifications must be provided for each sub-contractor or sub-grantee. Applicants requesting funding for multi-year grant programs are REQUIRED to provide a combined multi-year Budget Narrative/Justification, as well as a detailed Budget Narrative/Justification for each year of potential grant funding. A separate Budget Narrative/Justification is also REQUIRED for each potential year of grant funding requested.
For your use in developing and presenting your Budget Narrative/Justification, a sample format with examples and a blank sample template have been included in these Attachments. In your Budget Narrative/Justification, you should include a breakdown of the budgetary costs for all of the object class categories noted in Section B, across three columns: federal; non-federal cash; and non-federal in-kind. Cost breakdowns, or justifications, are required for any cost of $1,000 or more. The Budget Narratives/Justifications should fully explain and justify the costs in each of the major budget items for each of the object class categories, as described below. Non-federal cash as well as, sub-contractor or sub-grantee (third party) in-kind contributions designated as match must be clearly identified and explained in the Budget Narrative/Justification The full Budget Narrative/Justification should be included in the application immediately following the SF 424 forms. |
Line 6a: Personnel: Enter total costs of salaries and wages of applicant/grantee staff. Do not include the costs of consultants; consultant costs should be included under 6h ‑ Other. In the Budget Narrative/Justification: Identify the project director, if known. Specify the key staff, their titles, brief summary of project related duties, and the percent of their time commitments to the project in the Budget Narrative/Justification.
Line 6b: Fringe Benefits: Enter the total costs of fringe benefits unless treated as part of an approved indirect cost rate. In the Justification: Provide a break‑down of amounts and percentages that comprise fringe benefit costs, such as health insurance, FICA, retirement insurance, etc.
Line 6c: Travel: Enter total costs of out‑of‑town travel (travel requiring per diem) for staff of the project. Do not enter costs for consultant's travel - this should be included in line 6h. In the Justification: Include the total number of trips, destinations, purpose, and length of stay, subsistence allowances and transportation costs (including mileage rates).
Line 6d: Equipment: Enter the total costs of all equipment to be acquired by the project. For all grantees, "equipment" is non‑expendable tangible personal property having a useful life of more than one year and an acquisition cost of $5,000 or more per unit. If the item does not meet the $5,000 threshold, include it in your budget under Supplies, line 6e. In the Justification: Equipment to be purchased with federal funds must be justified as necessary for the conduct of the project. The equipment must be used for project-related functions; the equipment, or a reasonable facsimile, must not be otherwise available to the applicant or its sub‑grantees. The justification also must contain plans for the use or disposal of the equipment after the project ends.
Line 6e: Supplies: Enter the total costs of all tangible expendable personal property
(supplies) other than those included on line 6d. In the Justification: Provide general description of types of items included.
Line 6f: Contractual: Enter the total costs of all contracts, including (1) procurement
contracts (except those, which belong on other lines such as equipment, supplies, etc.). Also include any contracts with organizations for the provision of technical assistance. Do not include payments to individuals or consultants on this line. In the Budget Narrative/Justification: Attach a list of contractors indicating the name of the organization, the purpose of the contract, and the estimated dollar amount. If the name of the contractor, scope of work, and estimated costs are not available or have not been negotiated, indicate when this information will be available. Whenever the applicant/grantee intends to delegate more than 33% of a project’s total budget to the contractual line item, the applicant/grantee must provide a completed copy of Section B of the SF 424A Budget Categories for each sub-contractor or sub-grantee, and separate Budget Narrative/Justification for each sub-contractor or sub-grantee for each year of potential grant funding.
Line 6g: Construction: Leave blank since construction is not an allowable cost under this program.
Line 6h: Other: Enter the total of all other costs. Such costs, where applicable, may include, but are not limited to: insurance, medical and dental costs (i.e. for project volunteers this is different from personnel fringe benefits); non-contractual fees and travel paid directly to individual consultants; local transportation (all travel which does not require per diem is considered local travel); postage; space and equipment rentals/lease; printing and publication; computer use; training and staff development costs (i.e. registration fees). If a cost does not clearly fit under another category, and it qualifies as an allowable cost, then rest assured this is where it belongs. In the Justification: Provide a reasonable explanation for items in this category. For individual consultants, explain the nature of services provided and the relation to activities in the project. Describe the types of activities for staff development costs.
Line 6i: Total Direct Charges: Show the totals of Lines 6a through 6h.
Line 6j: Indirect Charges: Enter the total amount of indirect charges (costs), if any. If no indirect costs are requested, enter "none." Indirect charges may be requested if: (1) the applicant has a current indirect cost rate agreement approved by the Department of Health and Human Services or another federal agency; or (2) the applicant is a state or local government agency.
Budget Narrative/Justification: State governments should enter the amount of indirect costs determined in accordance with DHHS requirements. An applicant that will charge indirect costs to the grant must enclose a copy of the current indirect cost rate agreement. If any sub-contractors or sub-grantees are requesting indirect costs, copies of their indirect cost agreements must also be included with the application.
If the applicant organization is in the process of initially developing or renegotiating a rate, it should immediately upon notification that an award will be made, develop a tentative indirect cost rate proposal based on its most recently completed fiscal year in accordance with the principles set forth in the cognizant agency's guidelines for establishing indirect cost rates, and submit it to the cognizant agency. Applicants awaiting approval of their indirect cost proposals may also request indirect costs. It should be noted that when an indirect cost rate is requested, those costs included in the indirect cost pool should not also be charged as direct costs to the grant. Also, if the applicant is requesting a rate which is less than what is allowed under the program, the authorized representative of the applicant organization must submit a signed acknowledgement that the applicant is accepting a lower rate than allowed.
Line 6k: Total: Enter the total amounts of Lines 6i and 6j.
Line 7: Program Income: As appropriate, include the estimated amount of income, if any, you expect to be generated from this project. Program Income must be used as additional program costs and cannot be used as match (non-federal resource).
Section C ‑ Non‑Federal Resources
Line 12: Enter the amounts of non‑federal resources that will be used in carrying out the proposed project, by source (Applicant; State; Other) and enter the total amount in Column (e). Keep in mind that if the match requirement is not met, federal dollars may be reduced.
Section D ‑ Forecasted Cash Needs - Not applicable.
Section E ‑ Budget Estimate of Federal Funds Needed for Balance of the Project
Line 20: Section E is relevant for multi-year grant applications, where the project period is 24 months or longer. This section does not apply to grant awards where the project period is less than 17 months.
Section F ‑ Other Budget Information
Line 22: Indirect Charges: Enter the type of indirect rate (provisional, predetermined, final or fixed) to be in effect during the funding period, the base to which the rate is applied, and the total indirect costs. Include a copy of your current Indirect Cost Rate Agreement.
Line 23: Remarks: Provide any other comments deemed necessary.
c. Standard Form 424B - Assurances
This form contains assurances required of applicants under the discretionary funds programs administered by the Assistant Secretary for Preparedness and Response. Please note that a duly authorized representative of the applicant organization must certify that the organization is in compliance with these assurances.
d. Certification Regarding Lobbying
This form contains certifications that are required of the applicant organization regarding lobbying. Please note that a duly authorized representative of the applicant organization must attest to the applicant’s compliance with these certifications.
e. Other Application Components
Survey on Ensuring Equal Opportunity for Applicants
The Office of Management and Budget (OMB) has approved an HHS form to collect information on the number of faith-based groups applying for a HHS grant. Non-profit organizations, excluding private universities, are asked to include a completed survey with their grant application packet. Attached you will find the OMB approved HHS “Survey on Ensuring Equal Opportunity for Applicants” form (Attachment F). Your help in this data collection process is greatly appreciated.
Proof of Non-Profit Status
Non-profit applicants must submit proof of non-profit status. Any of the following constitutes acceptable proof of such status:
A copy of a currently valid IRS tax exemption certificate.
A statement from a State taxing body, State attorney general, or other appropriate State official certifying that the applicant organization has a non-profit status and that none of the net earnings accrue to any private shareholders or individuals.
A certified copy of the organization’s certificate of incorporation or similar document that clearly establishes non-profit status.
Indirect Cost Agreement
Applicants that have included indirect costs in their budgets must include a copy of the current indirect cost rate agreement approved by the Department of Health and Human Services or another federal agency. This is optional for applicants that have not included indirect costs in their budgets.
J.Budget Narrative/Justification, Page 1 – Sample Format with EXAMPLES
Below is an example of how to reflect project costs in the template provided., and are suggested to offer guidelines when applicants are completing their budget justifications. Justifications must include supporting detail and narrative justification for the costs proposed. Sufficient detail should be provided to demonstrate costs as they pertain to the administration of the project. In any case, the applicant should assure that the narrative and justification are legible and clearly provide all required information.
INSTRUCTIONS:
The Budget Detail must include the following information:
An itemized breakout of proposed costs and sub-total of these costs for each Object Class Category listed in the template below.
A breakout of proposed costs by whether they are funded through Federal, Non-Federal Cash or Non-Federal In-Kind support.
A brief description of the expense or service in the Justification column, as they demonstrate costs pertaining to the administration of the project.
The time period in which the cost will be utilized in the Justification column.
Any pertinent information that will aid the reviewer in evaluating the proposed cost.
The Budget Detail must be supported by a narrative justification of why the proposed costs are necessary and reasonable to fulfill the purpose and achieve the milestones of the proposed project, in context of the proposed technical approach. An example of such justification would be:
Project Administrator Salary Costs – assumes at least a master’s in public health or health administration, or equivalent degree, with at least 6 years’ experience managing health services, programs, or providers. Salary is typical for this level of qualifications and responsibility in the proposed service area. Assumes this position would provide executive-level direction and management oversight
Object Class Category |
Federal Funds |
Non-Federal Cash |
Non-Federal In-Kind |
TOTAL |
Justification |
|
Personnel |
$40,000 |
$5,000 |
|
$45,000 |
Project Administrator (name) = .3FTE @ $50,000/yr |
= $15,000 ($10,000 = Federal; $5,000 = Non-Federal) |
Project Director (name) = 1FTE @ $30,000 |
= $30,000 (Federal) |
|||||
TOTAL: |
$45,000 |
|||||
Fringe Benefits |
$12,600 |
0 |
0 |
$12,600 |
Fringes on Project Staff @ 28% of salary. (Federal) |
|
FICA (7.65%) |
= $ 3,442 |
|||||
Health (12%) |
= $ 5,400 |
|||||
Dental (5%) |
= $ 2,250 |
|||||
Life (2%) |
= $ 900 |
|||||
Workers Comp Insurance (.75%) |
= $ 338 |
|||||
Unemployment Insurance (.6%) |
= $ 270 |
|||||
TOTAL: |
$12,600 |
|||||
Travel |
$4,120 |
$1,547 |
|
$5,667 |
Travel to 2 Annual Grantee Meetings: |
(Federal) |
Airfare: 1 RT x 2 people x $750/RT x 2 |
= $3,000 |
|||||
Lodging: 2 nights x 2 people x $100/night x 2 |
= $ 800 |
|||||
Per Diem: 2 days x 2 people x $40/day x 2 |
= $ 320 |
|||||
TOTAL: |
$4,120 |
|||||
Out-of-Town Project Site Visits (Non-Federal cash) |
|
|||||
Car mileage: |
|
|||||
3 trips x 2 people x 350 miles/trip x $ .365/mile |
= $ 767 |
|||||
Lodging: |
|
|||||
3 trips x 2 people x 1 night/ trip x $50/night |
= $ 300 |
|||||
Per Diem: |
|
|||||
3 trips x 2 people x 2days/trip x $40/day |
= $ 480 |
|||||
|
TOTAL: |
$1,547 |
||||
Equipment |
0 |
0 |
0 |
0 |
No equipment requested |
|
|
||||||
Supplies |
$1,340 |
$2,160 |
|
$3,500 |
Laptop computer for use in client intakes |
= $1,340 (Federal) |
Consumable supplies (paper, pens, etc.) |
|
|||||
$100/mo x 12 months |
= $1,200 (Non-Federal cash) |
|||||
Copying $80/mo x 12 months |
= $ 960 (Non-Federal cash) |
|||||
TOTAL: |
$3,500 |
|||||
Contractual |
$150,000 |
|
$50,000 |
$200,000 |
Contracts to A,B,C direct service providers (name providers) |
|
contractor A |
= $75,000 (Federal) |
|||||
contractor B |
=$75,000 (Federal) |
|||||
contractor C |
=$50,000 (Non-Federal In-Kind) |
|||||
TOTAL: |
$200,000 |
|||||
Other |
$1,250 |
$2,000 |
|
$3,250 |
Local conf registration fee (provide conference name) |
= $ 200 (Non-Fed cash) |
Printing brochures (25,000 @ $0.05 ea) |
= $ 1,250 (Federal) |
|||||
Postage: $150/mo x 12 months |
= $ 1,800 (Non-Fed cash) |
|||||
TOTAL: |
$4,200 |
|||||
TOTAL |
$209,310 |
$10,707 |
$50,000 |
$270,017 |
|
|
K.Budget Narrative/Justification ––Template
Object Class Category |
Federal Funds |
Non-Federal Cash |
Non-Federal In-Kind |
TOTAL |
Justification |
Personnel |
|
|
|
|
|
Fringe Benefits |
|
|
|
|
|
Travel |
|
|
|
|
|
Equipment |
|
|
|
|
|
Supplies |
|
|
|
|
|
Contractual |
|
|
|
|
|
Other |
|
|
|
|
|
Indirect Charges |
|
|
|
|
|
TOTAL |
|
|
|
|
|
L.Instructions for Completing the Project Summary/Abstract
All applications for grant funding must include a Summary/Abstract that concisely describes the proposed project. It should be written for the general public.
To ensure uniformity, please limit the length to no more than 500 words on a single page with a font size of not less than 11, doubled-spaced.
The abstract must include the project’s goal(s), objectives, overall approach (including target population and significant partnerships), anticipated outcomes, products, and duration. The following are very simple descriptions of these terms, and a sample Compendium abstract.
Goal(s) – broad, overall purpose, usually in a mission statement, i.e. what you want to do, where you want to be.
Objective(s) – narrow, more specific, identifiable or measurable steps toward a goal. Part of the planning process or sequence (the “how”). Specific performances which will result in the attainment of a goal.
Outcomes - measurable results of a project. Positive benefits or negative changes, or measurable characteristics that occur as a result of an organization’s or program’s activities. (Outcomes are the end-point).
Products – materials, deliverables.
A model abstract/summary is provided below:
The grantee, Okoboji University, supports this three year Dementia Disease demonstration (DD) project in collaboration with the local Alzheimer’s Association and related Dementias groups. The goal of the project is to provide comprehensive, coordinated care to individuals with memory concerns and to their caregivers. The approach is to expand the services and to integrate the bio-psycho-social aspects of care. The objectives are: 1) to provide dementia specific care, i.e., care management fully integrated into the services provided; 2) to train staff, students and volunteers; 3) to establish a system infrastructure to support services to individuals with early stage dementia and to their caregivers; 4) to develop linkages with community agencies; 5) to expand the assessment and intervention services; 6) to evaluate the impact of the added services; 7) to disseminate project information. The expected outcomes of this DD project are: patients will maintain as high a level of mental function and physical functions (thru Yoga) as possible; caregivers will increase ability to cope with changes; and pre and post – project patient evaluation will reflect positive results from expanded and integrated services. The products from this project are: a final report, including evaluation results; a website; articles for publication; data on driver assessment and in-home cognitive retraining; abstracts for national conferences.
M.Survey instructions on Ensuring Equal Opportunity for Applicants

Applicant Organization’s Name: _________________________________________________
Applicant’s DUNS Number: ___________________
Grant Name: ____________________________________________________CFDA Number: _____________
Provide the applicant’s (organization) name and DUNS number and the grant name and CFDA number.
1. 501(c)(3) status is a legal designation provided on application to the Internal Revenue Service by eligible organizations. Some grant programs may require nonprofit applicants to have 501(c)(3) status. Other grant programs do not.
2. For example, two part-time employees who each work half-time equal one full-time equivalent employee. If the applicant is a local affiliate of a national organization, the responses to survey questions 2 and 3 should reflect the staff and budget size of the local affiliate.
3. Annual budget means the amount of money your organization spends each year on all of its activities.
4. Self-identify.
5. An organization is considered a community-based organization if its headquarters/service location shares the same zip code as the clients you serve.
6. An “intermediary” is an organization that enables a group of small organizations to receive and manage government funds by administering the grant on their behalf.
7. Self-explanatory.
8. Self-explanatory.
Paperwork Burden Statement
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless such collection displays a valid OMB control number. The valid OMB control number for this information collection is 1890-0014. The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have any comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: U.S. Department of Education, Washington, D.C. 2202-4651.
If you have comments or concerns regarding the status of your individual submission of this form, write directly to: Joyce I. Mays, Application Control Center, U.S. Department of Education, 7th and D Streets, SW, ROB-3, Room 3671, Washington, D.C. 20202-4725.
N.Glossary of Terms
EHR: For purposes of this Funding Opportunity Announcement “electronic health record”, “certified EHR" and “certified EHR technology” have been used interchangeably to signify electronic health record certified pursuant to Section 3001(c)(5) of the Public Health Service Act as added by the ARRA.
Health Information Exchange (HIE): For purposes of this Funding Opportunity Announcement, “Health Information Technology” or “HIE” is used to mean the electronic movement of health-related information among organizations according to nationally recognized standards.
Meaningful Use: Under the HITECH Act, an eligible professional or hospital is considered a "meaningful EHR user" if they use certified EHR technology in a manner consistent with criteria to be established by the Secretary through the rulemaking process, including but not limited to e-prescribing through an EHR, and the electronic exchange of information for the purposes of quality improvement, such as care coordination. In addition, eligible professionals and hospitals must submit clinical quality and other measures to HHS.
Pursuant to Titles 18 and 19 of the Social Security Act as amended by Title IV in Division B of ARRA, the Secretary will propose and finalize a definition for meaningful EHR use through formal notice-and-comment rulemaking by the end of FY 2010.
Provider Terms
Primary-Care Physician: For purposes of this Funding Opportunity Announcement, “Primary-Care Physician” is defined as a licensed doctor of medicine or osteopathy practicing family practice, obstetrics and gynecology, general internal or pediatric medicine regardless of whether the physician is board certified in any of these specialties.
Individual primary-care physician practice: For purposes of this Funding Opportunity Announcement, ”individual primary-care physician practice” is defined as a a practice in which only one primary-care physician furnishes professional services. The practice may include one or more nurse practitioners and/or physician assistants in lieu of or in addition to registered and licensed vocational nurses, medical assistants, and office administrative staff.
Small-group primary-care physician practice: For purposes of this Funding Opportunity Announcement, ”small-group primary-care physician practice” is defined as aa group practice site that includes 10 or fewer licensed doctors of medicine or osteopathy routinely furnish professional services, and where the majority of physicians practicing at least 2 days per week at the site practice family, general internal, or pediatric medicine. The practice may include nurse practitioners and/or physician assistants (regardless of their practice specialties) in addition to registered and licensed vocational nurses, medical assistants, and office administrative staff.
Note: a practice otherwise meeting the definition of individual or small-group physician practice, above, may participate in shared-services and/or group purchasing agreements, and/or reciprocal agreements for patient coverage, with other physician practices without affecting their status as individual or small-group practices for purposes of the Regional Centers.
Selected Definitions Relevant to the Medicare EHR Incentives
1886 (d) Hospitals: Section 1886(d) of the Social Security Act (the Act) sets forth a system of payment for the operating costs of acute care hospital inpatient stays under Medicare Part A (Hospital Insurance) based on prospectively set rates. This payment system is referred to as the inpatient prospective payment system (IPPS). Acute-care hospitals subject to IPPS 1886(d) are often referred to as 1886(d) hospitals.
Eligible Hospital: Per Title 18 of the Social Security Act as amended by Title IV in Division B of ARRA, an 1886(d) inpatient acute care hospital paid under the Medicare inpatient prospective payment system (IPPS) or an 1814(l) Critical Access Hospital (CAHs).
Non-eligible Hospital: Per Title 18 of the Social Security Act as amended by Title IV in Division B of ARRA, any hospital other than an acute-care hospital under 1886(d) or Critical Access Hospital under 1814(l). (Per SSA 1886(d), examples include Long-term Care Hospitals, Inpatient Rehabilitation Hospitals, Inpatient Psychiatric Hospitals, non-IPPS Cancer Centers and Children’s Hospitals.)
Eligible Professional: For purposes of the Medicare incentive, an eligible professional is defined in Social Security Act Section 1848(o), as added by ARRA, as a physician as defined in Social Security Act 1861(r). The definition at1861(r) includes doctors of medicine, doctors of osteopathy, doctors of dental surgery or of dental medicine, doctors of podiatric medicine, doctors of optometry, and chiropractors.
Hospital-Based Professional: SSA 1848(o)(1)(C)(ii), as added by ARRA, defines a ‘hospital-based professional’ for purposes of clause (i) of SSA 1848(o)(1)(C). A hospital-based professional is an otherwise eligible professional, such as a pathologist, anesthesiologist, or emergency physician, who furnishes substantially all of his or her covered professional services in a hospital setting (whether inpatient or outpatient) and through the use of the facilities and equipment, including qualified electronic health records, of the hospital. The determination of whether an eligible professional is a hospital-based eligible physician shall be made on the basis of the site of service (as defined by the Secretary) and without regard to any employment or billing arrangement between the priority primary care provider and any other provider. SSA 1848(o)(1)(C)(i) that no Medicare incentive payments for meaningful use of certified EHR technology may be made to hospital-based eligible professionals.
Selected Definitions Relevant to Medicaid EHR Incentives
Eligible professional: Social Security Act 1903(t)(3)(B), as added by ARRA, defines an eligible professional for Medicaid health IT incentives as a physician, dentist, certified nurse mid-wife, nurse practitioner, or a physician assistant practicing in a rural health clinic or FQHC that is led by a physician assistant, if he/she meets the criteria set forth in SSA 1903(t)(2)(A) as added by ARRA.
Rural Health Clinic: For purposes of this Funding Opportunity Announcement, “rural health clinic” is defined as a clinic providing primarily outpatient care certified to receive special Medicare and Medicaid reimbursement. RHCs provide increased access to primary care in underserved rural areas using both physicians and other clinical professionals such as nurse practitioners, physician assistants, and certified nurse midwives to provide services.
Federally Qualified Health Center (FQHC): A type of provider defined by the Medicare and Medicaid statutes for organizations that provide care to underserved populations and include Community Health Centers, Migrant Health Centers, Health Care for the Homeless Programs, Public Housing Primary Care Programs and some tribal clinics. FQHC provide services in both medically underserved area and to medically underserved populations.
Eligible Hospital: The definition of Medicaid providers for purposes of eligibility for Medicaid HIT incentive payments, provided at Social Security Act 1903(t)(2)(B), as added by ARRA, is a Children's Hospital or an Acute Care Hospital with at least 10 percent patient volume attributable to Medicaid.
Other Definitions for the purpose of this announcement
Note: Unless otherwise noted in the specific definition, the below terms are defined as used in this Funding Opportunity Announcement, for purposes of this announcement.
Health IT: certified EHRs and other technology and connectivity required to meaningfully use and exchange electronic health information
Priority primary care providers: Primary-care providers in individual and small group practices (fewer than 10 physicians and/or other health care professionals with prescriptive privileges) primarily focused on primary care; and physicians, physician assistants, or nurse practitioners who provide primary care services in public and critical access hospitals, community health centers, and in other settings that predominantly serve uninsured, underinsured, and medically underserved populations.
Provider: All providers included in the definition of “Health Care Provider” in Section 3000(3) of the Public Health Service Act (PHSA) as added by ARRA. This includes, though it is not limited to, hospitals, physicians, priority primary care providers, Federally Qualified Health Centers (and “Look-Alikes”) and Rural Health Centers.
Primary-care physician: A licensed doctor of medicine (MD) or osteopathy (DO) who practices family, general internal or pediatric medicine or obstetrics and gynecology.
Primary-Care Provider: A primary-care physician or a nurse practitioner, nurse midwife, or physician assistant with prescriptive privileges in the locality where s/he practices and practicing in one of the specialty areas included in the definition of a primary-care physician for purposes of this announcement.
Shared Directory: A service that enables the searching and matching of data to facilitate the routing of information to providers, patients and locations.
1 The announcements and start dates are approximate.
2 Definitions are detailed in Section I.F.4(Consensus Definitions).
3 Barriers and enablers include but are not limited to the following categories: technical, legal, financial, organizational.
4 The NHIN defines the essential components and provides an operational infrastructure necessary for nationwide health information exchange including standards, specifications, implementation guidelines, policies, and trust agreements.
5 ONC recognizes there may be state Strategic Plans that are already complete, currently being drafted or undergoing modification. ONC is not asking for a full restructuring of these plans, but rather that a state communicate and demonstrate that the required sections are covered.
6 While implementation funding may not be available at award if plans are not complete or consistent with program guidance, implementation funding will be available at the agreed-upon milestone (which includes approval of plans consistent with program guidance).
7 This award floor applies to states, the District of Columbia, and the Commonwealth of Puerto Rico. The amount for remaining Territories will be determined based on population size and needs.
8 While the total number could be 56 awards, it is anticipated that multi-state or multi-state-territory applications will be submitted such that the number of awards is estimated to be approximately 50.
9 For purposes of this program agreement, unless otherwise indicated “state” also includes the District of Columbia and the U.S. territories – Puerto Rico, U.S. Virgin Islands, Guam, the Northern Mariana Islands, and American Samoa.
10 For applicants awaiting not-for-profit status determination, ONC will work individually with these applicants on a case by case basis.
11 For state agency applicants, alternative methods for governance will be considered to ensure adequate mechanisms exist for multi-stakeholder input, public accountability, and oversight of health information exchange.
12 ONC will negotiate with each state to determine best way to further specify this measure based on the statewide directories and shared services pursued within each state under this program.
13 Formal Application includes the preliminary application and letter of intent phases of the program.
| File Type | application/msword |
| File Title | State Health Information Exchange Cooperative Agreement Program |
| Subject | State Health Information Exchange Cooperative Agreement Program |
| Author | ONC Grants |
| Last Modified By | Seleda.Perryman |
| File Modified | 2009-09-04 |
| File Created | 2009-09-04 |
© 2025 OMB.report | Privacy Policy