Guidance 152
guidance 152.doc
New Animal Drug Application, 21 CFR Part 514
Guidance 152
OMB: 0910-0032
# 152
Guidance for Industry
Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern
This document discusses a recommended approach for assessing the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern.
Comments and suggestions regarding this document should be sent to the Division of Dockets Management (HFA 305), Food and Drug Administration, 5630 Fishers Lane, Room 1061, Rockville, MD 20852. All comments should be identified with the Docket No.98D-1146. Submit electronic comments to http://www.fda.gov/dockets/ecomments.
Direct questions regarding this document to Jeffrey M. Gilbert, (HFV-157), Center for Veterinary Medicine, Food and Drug Administration, 7500 Standish Place, Rockville, MD 20855, 301-827-0233, e-mail: jgilbert@cvm.fda.gov.
Additional copies of this guidance document may be requested from the Communications Staff (HFV-12), Center for Veterinary Medicine, Food and Drug Administration, 7519 Standish Place, Rockville, MD 20855 and may be viewed on the Internet at http://www.fda.gov/cvm.
Paperwork Reduction Act Public Burden Statement
According to the Paperwork Reduction Act of 1995, a collection of information should display a valid OMB control number. The valid OMB control number for this information collection is 0910-0032 (Expiration Date ). The time required to complete this information collection is estimated to average 90 hours per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection.
U .S.
Department of Health and Human Services
.S.
Department of Health and Human Services
Food and Drug
Administration
Center for Veterinary Medicine
October 23,
2003

Table of Contents
I. Introduction 2
II. Scope of Guidance 3
III. Risk Analysis Methodology 5
IV. Hazard Characterization 8
V. Qualitative Antimicrobial Resistance Risk Assessment 9
A. Release Assessment 10
B. Exposure Assessment 14
C. Consequence Assessment 20
D. Risk Estimation 20
VI. Antimicrobial Resistance Risk Management Considerations 22
VII. Application of Risk Management Strategies 24
VIII. Summary of Microbial Food Safety Assessment Process 26
Glossary 27
Appendix A: Ranking of antimicrobial drugs according to their importance in human medicine 28
References 34
Evaluating the Safety of Antimicrobial New Animal Drugs With Regard to Their Microbiological Effects on Bacteria of Human Health Concern1
This guidance represents
the Food and Drug Administration’s (FDA’s) current
thinking on this topic. It does not create or confer any rights for
or on any person and does not operate to bind FDA or the public.
You can use an alternative approach if the approach satisfies the
requirements of the applicable statute and regulations. If you want
to discuss an alternative approach, contact the FDA staff
responsible for implementing the guidance. If you cannot identify
the appropriate staff, call the appropriate number listed on the
title page of this guidance.
Prior to approving an antimicrobial new animal drug application, FDA must determine that the drug is safe and effective for its intended use in the animal. The Agency must also determine that the antimicrobial new animal drug intended for use in food-producing animals is safe with regard to human health (21 CFR 514.1(b)(8)). FDA considers an antimicrobial new animal drug to be “safe” if it concludes that there is reasonable certainty of no harm to human health from the proposed use of the drug in food-producing animals. This document provides guidance for industry on a possible process for evaluating the potential effects of antimicrobial new animal drugs on non-target bacteria as part of the new animal drug application process.
This guidance document outlines a risk assessment approach for evaluating the microbial food safety of antimicrobial new animal drugs. Within the context of risk assessment, many possible mechanisms to address the development of antimicrobial resistance resulting from the use of antimicrobial new animal drugs in food-producing animals are available to the sponsor. Alternative processes that may be more appropriate to a sponsor’s drug and its intended conditions of use, may be used to characterize the microbial food safety of that drug.
FDA’s guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as guidance, unless specific regulatory or statutory requirements are cited. The use of the word “should” in Agency guidances means that something is suggested or recommended, but not required.
II. Scope of Guidance Document
As part of the pre-approval safety evaluation process, FDA intends to consider the potential impact on human health of all uses of all classes of antimicrobial new animal drugs intended for use in food-producing animals. The scope of this document is an assessment of the effect of the transmission of foodborne bacteria of human health concern through the consumption of animal derived food products. Although FDA’s primary focus will be foodborne pathogens, other (enteric/gastrointestinal) bacteria may be considered when deemed necessary.
Further clarification is provided regarding microbial food safety considerations that should be addressed, and the investigational new animal drugs (INADs) or new animal drug applications (NADAs) covered by the guidance described herein. This document focuses on the concern that the use of antimicrobial new animal drugs in food-producing animals will result in the emergence and selection of antimicrobial resistant food-borne bacteria which impact human health adversely.
Note: Effects of drug residues on human intestinal microflora: Antimicrobial drug residues present in food from food-producing animals may cause adverse effects on the ecology of the intestinal microflora of consumers.1, 2 For further information on requirements regarding these effects, refer to FDA Guidance for Industry #52 entitled “Assessment of the Effects of Antimicrobial Drug Residues from Food of Animal Origin on the Human Intestinal Flora.”
The FDA believes that human exposure through the ingestion of antimicrobial resistant bacteria from animal-derived foods represents the most significant pathway for human exposure to bacteria that have emerged or been selected as a consequence of antimicrobial drug use in animals.
This risk assessment approach is recommended for all uses of all antimicrobial new animal drugs in food‑producing animals; however, sponsors of applications described below are encouraged to consult with FDA to decide if the risk assessment approach is recommended for their application.
1. Certain supplemental NADAs: Microbial food safety information is not typically needed for Category I supplemental NADAs (21 CFR 514.106(b)(1)). These supplements ordinarily do not require a reevaluation of any of the safety or effectiveness data in the parent application. However, information may be needed for certain Category II supplemental NADAs (21 CFR 514.106(b)(2)). These supplements may require a re-evaluation of certain safety or effectiveness data in the parent application.
2. NADAs for antimicrobial drug combinations: Microbial food safety information would ordinarily not be needed for antimicrobial drug combinations as defined in Section 512(d) of the Act (21 U. S. C. 360b(d)), as amended by the Animal Drug Availability Act (ADAA) of 1996. Microbial food safety would typically be addressed as part of the NADAs for the individual antimicrobial drugs that comprise the combination. However, in certain circumstances information may be requested for drug applications for antimicrobial drug combinations.
3. Abbreviated (generic) NADAs: Microbial food safety information would not be needed for abbreviated new animal drug applications (ANADAs) filed under section 512(b)(2) of the Act for generic copies of approved antimicrobial new animal drugs. Microbial food safety information would be needed for supplements to add claims to approved ANADAs.
III. Risk Analysis Methodology
This guidance document outlines a risk analysis method, and describes its application as a process for evaluating human food safety with respect to the potential microbiological effects of antimicrobial new animal drugs on food-borne bacteria of human health concern. The sponsor of an antimicrobial new animal drug may use this guidance and the methodology described herein to conduct a qualitative risk assessment as part of the pre-approval safety evaluation of a new animal drug. It is important to note that the sponsor is free to demonstrate the safety of their proposed drug product in other ways.
FDA’s current thinking on a qualitative approach for risk assessment, especially where there may be a lack of substantial data, is described in this guidance. FDA does not intend to exclude quantitative risk assessment in favor of a qualitative process. Further, FDA encourages sponsors to seek data and modeling approaches that can best refine and improve the approach and assumptions incorporated in this risk assessment process.
If the sponsor elects to use this or a similar process, FDA recommends the assessment be submitted to the INAD file with supporting data as a component of the Human Food Safety technical section, or should be included in the NADA as part of the sponsor’s submission under 21 CFR 514.1(b)(8). The results of this risk assessment can help to estimate the overall risk, allowing an informed risk management decision. Evaluation of all available information submitted in support of the NADA may result in actions ranging from approval of the new animal drug to denial of the new animal drug application. The remainder of the document provides guidance on this risk analysis method.
A. Background:
The risk analysis process outlined in this document is based on the process described by the Office International des Epizooties (OIE) Ad Hoc Group on Antimicrobial Resistance.3 The OIE risk analysis methodology is tailored to address antimicrobial resistance in animals and includes hazard identification, risk assessment, risk management, and risk communication. Although the OIE approach differs organizationally from the risk analysis paradigm described by the National Academy of Science/National Research Council (NAS/NRC), the OIE process includes similar steps to describe the risk assessment.4
The risk assessment process described in this guidance is comprised of a hazard characterization, a release assessment, an exposure assessment, a consequence assessment, and a risk estimation (See Figure 1). The risk estimation integrates the components of the risk assessment into an overall conclusion, providing a qualitative indication of the potential risk to human health of the proposed use of the antimicrobial new animal drug. FDA then uses the overall risk estimation ranking, along with other relevant data and information submitted in support of the NADA, to determine whether the drug is approvable under specific risk management conditions.
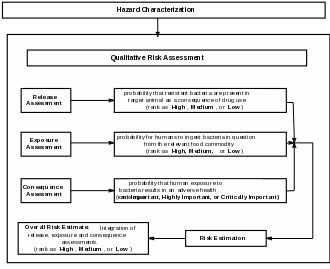
Figure 1: Components of a qualitative antimicrobial resistance risk assessment
B. Definitions:
1. Hazard: Human illness, caused by an antimicrobial-resistant bacteria, attributable to an animal-derived food commodity, and treated with the human antimicrobial drug of interest.
2. Hazardous agent: Antimicrobial-resistant food-borne bacteria of human health concern that are in or on a food-producing animal as a consequence of the proposed use of the antimicrobial new animal drug.
3. Risk: The probability that human food-borne illness is caused by an antimicrobial- resistant bacteria, is attributable to an animal-derived food commodity, and is treated with the human antimicrobial drug of interest.
FDA’s overriding concern is the decreased or lost effectiveness of antimicrobial drugs in humans as a consequence of human exposure to resistant bacteria through ingestion of animal derived food products. FDA is concerned about a range of deleterious effects that antimicrobial resistant bacteria may have on human health. These effects include but are not limited to increased duration of illness, treatment failure, and loss of therapeutic options. Due to the difficulties associated with measuring loss of effectiveness, the risk assessment process described in this guidance document estimates the probability of the occurrence of the hazard.
C. Data sources/data quality:
A variety of materials may be used to support a microbial food safety assessment. These materials should meet FDA standards for data used to support an approval. Sponsors may consider:
Generating necessary data through the conduct of prospective studies. FDA recommends that drug sponsors refer to 21 CFR Part 58 for requirements related to Good Laboratory Practices for conducting non-clinical laboratory studies.
Submission of current and relevant literature (including peer reviewed, published literature). FDA recommends that sponsors refer to Guidance for Industry #106, “The Use of Published Literature in Support of New Animal Drug Approval” for guidance regarding use of published literature.
Note: Prior to initiating and submitting the risk assessment, FDA recommends that sponsors electing to use this process characterize the hazard, and the conditions that influence the occurrence of that hazard. CVM envisions hazard characterization as distinct and separate from the qualitative risk assessment and it is recommended that the hazard characterization be submitted to the FDA as a stand alone document. This submission will enable the sponsor and the FDA to determine the information that should be included in the risk assessment. In addition, based on the hazard characterization, it may be determined in certain cases that completion of a risk assessment is not recommended.
The hazard has been defined as human illness, caused by an antimicrobial-resistant bacteria, attributable to an animal-derived food commodity, and treated with the human antimicrobial drug of interest.
FDA recommends that sponsors address the hazard characterization step of the risk assessment by submitting information regarding the chemical, biochemical, microbiological, and physical properties of the antimicrobial new animal drug that bear on characterizing the downstream effects of the drug. This information may include, but should not be limited to:
A. Drug-specific information:
Chemical name and structure
Class of antimicrobial drug (e.g., macrolide)
Mechanism (e.g., protein synthesis inhibitor) and type of action (i.e., bactericidal vs. bacteriostatic)
Spectrum of activity (e.g., Gram-positive, Gram-negative, broad, or narrow spectrum, etc.)
4. Standardized antimicrobial susceptibility testing methodology and specific susceptibility data (i.e., minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) data pertinent to the appropriate bacteria of human health concern). FDA recommends that if the sponsor does not use standardized susceptibility test methods, the sponsor should include a detailed description of the antimicrobial susceptibility testing method(s) used for determining the susceptibility of the bacterial isolates of concern and the reason(s) for the needed change. The methods should include the quality control organism(s), the dilution scheme used, and the source for the interpretive criteria for human or veterinary isolates. The methods may include citations, if available, of relevant laboratory standards such as the National Committee on Clinical Laboratory Standards (NCCLS). Additional guidance on susceptibility testing may be obtained from recognized sources such as NCCLS documents.
5. Relative importance of the drug in human medicine (see Appendix A).
B. Bacterial resistance information:
Taking into account the target animal species to be treated with the drug, the conditions of intended animal use of the drug in animals, and the antimicrobial properties of the drug in question, FDA recommends that the sponsor identify:
Bacterial species and strains for which resistance acquisition has potential human health consequence.
Known resistance determinants or mechanisms associated with the antimicrobial drug(s) of interest. FDA recommends that information describing phenotypic and genotypic similarities with resistance determinants in other food-borne bacteria of human concern be identified.
C. Data gaps and emerging science: The sponsor or FDA may identify data gaps and areas of emerging science that may be relevant to the microbial food safety assessment for the proposed conditions of use.
V. Qualitative Risk Assessment
Note: After submission and review of the hazard characterization, and prior to completing the risk assessment, the sponsor may wish to consult with FDA regarding recommendations on additional information to complete the risk assessment.
The OIE method is described below in a simplified format. The risk assessment approach is comprised of a release assessment, an exposure assessment, a consequence assessment, and a risk estimation (refer to Figure 1).
FDA recommends that sponsors adapt and expand their risk assessment to accommodate the unique relationships that may exist among an antimicrobial new animal drug, affected microbe(s), proposed condition(s) of use, and other parameters that potentially affect human health. The assessment process outlined below will result in an overall estimate of the level of concern (risk estimation) associated with the emergence or selection of resistant bacteria as a consequence of the proposed use of the drug in animals. This process may help guide the selection of appropriate risk management steps.
Note: FDA intends to determine the appropriate use conditions or other risk management steps based on its review and consideration of the new animal drug application as a whole, including any risk assessment submitted by the sponsor as part of the application.
Release Assessment:
The release assessment estimates the probability that the proposed use of the antimicrobial new animal drug in food-producing animals will result in the emergence or selection of resistant bacteria in the animal.
1. Defining the boundaries of the release assessment:
The boundaries of the release assessment span from the point the antimicrobial new animal drug is administered to the food-producing animal, to the point the animal is presented for slaughter or the animal-derived food is collected.
For the purposes of this guidance, FDA is focusing on the food-producing animal as the source of human exposure to the hazardous agent. Human exposure to the hazardous agent should be addressed in the exposure assessment.
2. Factors that may be considered in release assessment:
A number of relevant factors are suggested for consideration in completing the release assessment. These factors include items that are also considered as part of the hazard characterization step described earlier.
Note: Following submission of the hazard characterization, the sponsor may wish to consult with FDA to determine the specific factors most relevant to the proposed conditions of use of the antimicrobial new animal drug in question.
In order to address specific considerations pertinent to the drug and its proposed conditions of use, the sponsor or FDA may consider factors not listed below. The relative significance of any particular factor may vary depending on the specific antimicrobial new animal drug application under consideration. Therefore, when determining the overall release assessment ranking, certain factors may carry greater weight than other factors. FDA recommends that the factors considered in the release assessment include the following. Other factors may also be relevant. FDA recommends these be clearly defined and supported.
a. Product description:
Product formulation (active and inactive ingredients)
Information regarding proposed conditions of use including:
Route of administration (i.e., injection, water, feed)
Dosing regimen
Proposed product indication
Intended target animal species
Proposed withdrawal time
b. Drug substance description:
Class of antimicrobial drug (e.g., macrolide)
Chemical name, CAS number, and structure
c. Mechanism and type of antimicrobial action:
Specifics regarding antimicrobial mechanisms (e.g., protein synthesis inhibitor)
Type of action (e.g., bactericidal action vs. bacteriostatic)
d. Spectrum of activity:
General information (e.g., is active against Gram-positive, Gram-negative, broad, or narrow spectrum, etc.)
Specific susceptibility data (e.g., minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) data pertinent to the food-borne bacteria of human concern in question)
e. The pharmacokinetics/pharmacodynamics of the drug:
absorption, distribution, metabolism, and elimination of the drug in the target animal
data on, or an estimation of, the active antimicrobial drug in colonic contents
additional effects such as first-exposure effects, post-antibiotic effects, sub-MIC effects, etc.
Pharmacodynamics, such as concentration and/or time dependent effects, etc.
f. Resistance mechanisms and genetics: FDA recommends that the sponsor provide information regarding the mechanism(s) and genetic basis of resistance development that includes:
Known mechanism(s) of resistance in animal and human pathogens (e.g., antimicrobial inactivation, alteration of the drug target, reduced uptake, efflux of the antimicrobial drug, etc.)
Location of resistance determinants (e.g., plasmid-mediated vs. chromosomal; present on transposon, integron, or phage)
g. Occurrence and rate of transfer of resistance determinants: FDA recommends that the sponsor provide information regarding whether resistance determinants are transferable and, if so, at what rate. Relevant questions may include, but are not limited to:
Can resistance determinants be transferred among bacteria by transformation, transduction, conjugation, or transposition? If so, at what rate?
If resistance occurs by point mutation, at what rate do the point mutations occur?
h. Resistance selection pressures: FDA recommends that the sponsor provide information to help characterize the relative magnitude of selection pressure for resistance that may exist for the particular drug use in question. Pertinent information may include:
Information regarding other antimicrobials that may co-select for resistance
Information regarding cross resistance to other antimicrobial drugs approved in veterinary and human medicine
Consideration of the extent of use of the proposed product (e.g., duration of administration; individual vs. small groups vs. flocks/herds)
i. Baseline prevalence of resistance: FDA recommends that the sponsor provide available epidemiological data outlining the existing prevalence of resistance to the drug and/or related drugs in target pathogens and commensal gut flora. This may be obtained from newly generated data, or existing sources of data, such as the National Antimicrobial Resistance Monitoring System (NARMS) data, current literature, or other reliable surveillance sources. If baseline data is not available for the proposed antimicrobial drug, sponsors may wish to consult with FDA regarding collection or generation of such data.
j. Other information relevant to the release assessment:
Relevant information relating to the rate of resistance development and decline after treatment
Information or studies to characterize the rate of resistance development in food-borne bacteria of human health concern following use of the drug under the proposed conditions of use.
Information or studies to characterize the decline of resistance in food-borne bacteria of human health concern following cessation of therapy. Of particular interest is information relative to the interval up to the earliest time point (post-drug administration) at which animals would be presented for slaughter.
3. Summarizing the Release Assessment:
FDA recommends that the sponsor qualitatively characterize all factors relevant to the release assessment based on supporting information. We recommend that this characterization include an estimate of whether each factor would have a high, medium, or low likelihood of favoring resistance emergence. For example, the spectrum of activity of the drug might be ranked high for favoring resistance emergence or selection if the new animal drug in question readily selects for mutations conferring resistance; in contrast, pharmacodynamics might be ranked low with regard to impact on resistance if the drug did not enter the target animal intestinal tract at concentrations shown to have an effect on resistance development, etc. These rankings would then be integrated into an overall release assessment ranking of high, medium, or low. FDA recommends that the sponsor provide a detailed discussion of the conclusions as well as present the conclusions in summary format (see Table 1).
Note: If sufficient information regarding a factor is not available or has not been generated for the assessment, the most conservative estimate (high) of the particular factor should be assumed.
Table 1: Sample table for collating and summarizing interpretation of relevant factors considered in completing the release assessment
-
Relevant parameters
Extent to which relevant factors favor emergence of resistance
Release2
(H, M, L)
Comments/conclusions regarding factors
Mechanism of activity
Spectrum of activity
Pharmacokinetics
Pharmacodynamics
Resistance mechanism(s)
Resistance transfer
Selection pressure
Other factors1
1Other factors may be identified that are thought to be of importance to the evaluation. After submission of the hazard characterization, the sponsor may wish to consult with FDA regarding additional factors prior to completing the assessment.
2Potential for favoring the release of resistant bacteria.
4. Release Assessment conclusion:
The outcome of the release assessment is intended to estimate the probability that resistant bacteria will emerge or be selected for as a consequence of the proposed drug use in animals. FDA recommends that the sponsor use the conclusions obtained from assessing all relevant factors to derive an overall qualitative ranking for the release assessment. This overall conclusion may be expressed in terms of a high, medium, or low probability that resistant food-borne bacteria will occur in animals as a consequence of the proposed drug use.
The exposure assessment describes the likelihood of human exposure to food-borne bacteria of human health concern through particular exposure pathways, in this case animal derived food products. The exposure assessment should provide a qualitative estimate of the probability of this exposure occurring.
The division of the qualitative risk assessment into “release” and “exposure” components effectively produces a natural placement of animal and animal treatment factors into the “release assessment component” and food-chain and human factors within the “exposure assessment component.” FDA recognizes that there are many factors that may affect the bacteria of interest between the time animals are presented for slaughter (or the animal-derived food is collected) and the time the final food product is consumed.
Note: For the purposes of this qualitative risk assessment, FDA assumes that the probability that bacteria in or on the animal at slaughter may be used as an estimate of the probability of human exposure to that bacterial species in the food commodity derived from that animal.
FDA recognizes that food-borne human exposure to antimicrobial resistant bacteria is complex and often involves the contributions from other sources of exposure (e.g., direct contact between animals and humans, introduction of resistant bacteria and resistance determinants into the environment). However, FDA believes that evaluating antimicrobial new animal drug safety relative to the most significant exposure pathway (i.e., food-borne pathway) is the best way to qualitatively assess the risk of antimicrobial drug use in food-producing animals. Uncertainties regarding the contribution of other exposure pathways may be considered during the development of appropriate risk management strategies.
1. Factors to consider in the exposure assessment:
The exposure assessment is independent of the use of the antimicrobial drug under review and may be estimated by considering the relative amount of relevant bacterial contamination of the food product and the relative quantity of the food product consumed by humans. While it is acknowledged that other factors such as food preparation practices can affect exposure, the two prior considerations are intended to provide a qualitative indication of the probability of human exposure to the food-borne bacteria of human health concern. Appropriate current survey data of both food commodity contamination and consumption may be submitted to support a qualitative ranking of the probability of human exposure to the given bacteria via a particular food commodity.
FDA recommends that the sponsor derive the exposure assessment ranking by integrating the ranking of the probability of human exposure (through food) to the bacteria in question with the ranking of consumption of the animal derived food commodity. The qualitative probability should be expressed in terms of high, medium, or low as discussed below.
2. Example process for the estimation of exposure to the hazardous agent:
Note: The specific information provided in the tables in this section is for illustrative purposes only. Sponsors may reference a variety of data sources which best characterize human exposure to bacteria of human health concern via animal-derived foods. FDA recommends that sponsors reference the most reliable, current data available at the time that the assessment for their product is conducted.
FDA believes that the concept of qualitatively ranking bacterial contamination in the manner described is consistent with the overall risk assessment process outlined. In addition, FDA believes that the incidence of carcass contamination is a relevant factor in estimating the probability of human exposure to foodborne bacteria. For the purposes of this risk assessment, FDA assumes that a high incidence of carcass contamination is more likely to lead to human exposure through food than a low incidence of carcass contamination. Based on this assumption, FDA believes that it is appropriate to rank contamination qualitatively as high, medium, or low.
Food commodity consumption: As an example of food commodity consumption data, per capita meat consumption data are provided in Table 2. The data presented are for the year 2001 and are published by the USDA Economic Research Service. FDA recommends that the sponsor reference this type of information when completing the risk assessment for their product. The most recent available information should be used for the assessment. The qualitative rankings provided in Table 2 are illustrative, and represent relative rankings of consumption of the commodities listed for the year 2001.
Table 2: Per capita consumption data for red meats, poultry, fish and shellfish for the year 2001.
-
Commodity
Per capita consumption*
(pounds per capita per year)Qualitative ranking**
Beef
62.9
High
Chicken
53.9
High
Pork
46.7
High
Fish and shellfish
15.2
Medium
Turkey
13.7
Medium
Lamb and mutton
0.8
Low
Veal
0.5
Low
Total meat
193.7
*From USDA Economic Research Service5; Boneless, trimmed (edible) weight.
**Qualitative ranking based on relative proportion of the total per capita consumption of meat that is attributable to each of the individual meat commodities.
Food commodity contamination: FDA recommends that the sponsor reference food commodity contamination data when completing the risk assessment for their product. The most recent information should be used for the assessment. The relative qualitative ranking of the level of contamination among various food commodities, High (> 25%), Medium (5–25%), Low (< 5%), is a general ranking, proposed here for illustrative purposes only, and may be subject to modification to more appropriately reflect the most current data.
For illustrative purposes, Tables 3 and 4 present Salmonella and Campylobacter contamination rates in various animal-derived food commodities.
Table 3. Prevalence of Salmonella contamination of various animal-derived food commodities and qualitative contamination rankings.
Commodity |
Baseline prevalence (%)1 |
Calendar Year 2001 Prevalence (%)1,2 |
Qualitative ranking3 |
Ground Turkey |
49.9 |
26.2 |
High |
Ground Chicken |
44.6 |
19.5 |
Medium |
Broilers |
20.0 |
11.9 |
Medium |
Market hog |
8.7 |
3.8 |
Low |
Ground Beef |
7.5 |
2.8 |
Low |
Cows/bulls |
2.7 |
2.4 |
Low |
Steer/Heifer |
1.0 |
0.6 |
Low |
1As reported in the USDA/FSIS “Progress Report on Salmonella Testing of Raw Meat and Poultry Products, 1998-2001”6
2Prevalence data for CY 2001 for all size slaughter establishments and establishments that produce raw ground product
3Relative qualitative ranking of the level of contamination among various food commodities, Low (< 5%), Medium (5 – 25%), High (> 25%), is a general ranking, proposed here for illustrative purposes only, and may be subject to modification to more appropriately reflect the most current data.
Table 4. Prevalence of Campylobacter contamination of various animal-derived food commodities and provisional qualitative contamination rankings.
Commodity |
Prevalence (%)1 |
Qualitative ranking2 |
Turkeys |
90 |
High |
Broilers |
88 |
High |
Ground Chicken |
60 |
High |
Market hog |
32 |
High |
Ground Turkey |
25 |
Medium |
Steer/Heifer |
4 |
Low |
Cows/bulls |
1 |
Low |
Ground Beef |
0 |
Low |
1Data from national surveys conducted between 1992 – 1997.7-14
2Relative qualitative ranking of the level of contamination among various food commodities; Low (< 5%), Medium (5–25%), High (> 25%) is a general ranking, proposed here for illustrative purposes only, and may be subject to modification to more appropriately reflect the most current data.
FDA acknowledges that the calendar year 2001 contamination data listed in Table 3 indicate that all listed food commodities are below their respective Salmonella performance standards (i.e., baseline prevalence). For the purposes of the assessment outlined here, FDA has decided to base the criterion for “high” contamination upon the highest level of contamination reported for Salmonella in 2001. Therefore, for the year 2001, a prevalence of contamination of greater than 25 percent is considered a “high” level of contamination. The medium and low rankings of contamination are bracketed at 5 to 25 percent and less than 5 percent, respectively. For consistency, as described in Table 4, the same ranking criteria may be applied to other bacteria such as Campylobacter. Sponsors may propose alternative criteria and rankings, if data are available to support their position.
3. Summarizing exposure assessment: Ranking human exposure to foodborne bacteria.
Table 5 describes a possible process for estimating the probability of human exposure to the hazardous agent through consumption of animal derived food commodities.
Table 5: Possible process for ranking qualitatively the probability of human exposure to a given bacteria in a given food commodity
-
Probability of human exposure to a given bacteria
Amount of food commodity being consumed
Amount of food commodity contamination
High
Medium
Low
High
H
H
M
Medium
H
M
L
Low
M
L
L
4. Exposure assessment conclusion
The outcome of the exposure assessment is intended to estimate the probability that humans will be exposed to the hazardous agent through consumption of animal derived food commodities. FDA recommends that the sponsor use the outcome of the integration process described in Table 5 to reach an overall qualitative rank of a high, medium, or low probability of human exposure to the hazardous agent.
FDA believes that the potential human health consequences of exposure to the defined hazardous agent may be qualitatively estimated by considering the human medical importance of the antimicrobial drug in question.
While antimicrobial agents are important for the treatment of infectious disease in humans, certain antimicrobial agents are believed to be of greater importance to the therapy of infectious diseases in humans than are others. Therefore, it is assumed that the human health consequences associated with bacteria that are resistant to drugs of greater importance are more significant than the consequences associated with bacteria that are resistant to drugs of lesser importance.
FDA recommends the sponsor refer to Appendix A of this document to assess the importance of the drug or antimicrobial class in question for human medicine. FDA recommends that the sponsor base the consequence assessment conclusion on the human medical importance ranking and be expressed as critically important, highly important or important. This ranking will be integrated along with the outcomes of the release and exposure assessments to derive an overall risk estimation as described below.
The risk estimation integrates the results from the release, exposure, and consequence assessments into an overall risk estimation associated with the proposed conditions of use of the drug. FDA recommends that the risk estimation rank drugs as high, medium, or low risk. The risk rankings represent the potential for human health to be adversely impacted by the selection or emergence of antimicrobial resistant food-borne bacteria associated with the use of the drug in food-producing animals.
Table 6 provides a possible method for integrating the outcomes of the release, exposure, and consequence assessments into a single risk estimation ranking. The distribution of risk estimation rankings listed in Table 6 provides an initial indication as to the integration of rankings. Refinement of the risk estimation ranking may be appropriate for specific cases based on available information.
Table 6. Possible risk estimation outcomes based on the integration of the release, exposure, and consequence assessment rankings
-
Release
Exposure
Consequence
Risk Estimation
low
low
important
low
low
medium
important
low
medium
low
important
low
low
low
highly important
low
low
high
important
medium
high
low
important
medium
medium
medium
important
medium
medium
high
important
medium
high
medium
important
medium
high
high
important
medium
low
medium
highly important
medium
low
high
highly important
medium
medium
medium
highly important
medium
medium
low
highly important
medium
medium
high
highly important
medium
high
low
highly important
medium
high
medium
highly important
medium
low
low
critically important
high
high
high
highly important
high
low
medium
critically important
high
medium
low
critically important
high
low
high
critically important
high
high
low
critically important
high
medium
medium
critically important
high
medium
high
critically important
high
high
medium
critically important
high
high
high
critically important
high
VI. Risk Management Considerations
Possible risk management steps range from denying the approval of a drug application (i.e., the drug is unsafe or not shown to be safe) to approving the application under various use conditions that assure the safe use of the product.
Denying approval of a drug application: The Federal Food, Drug, and Cosmetic Act (FFDCA), Sec. 512(d), and regulations promulgated thereunder (see 21 CFR 514.111), provides possible grounds for denying the approval of a new animal drug application. The statutory grounds for denying approval include the results of tests that show the drug is unsafe or the determination that there is insufficient information as to whether the drug is safe. Consequently, denying the approval of an antimicrobial drug application is one possible outcome of an overall safety evaluation which could include the qualitative antimicrobial resistance risk assessment process described above.
Drug approval under safe conditions of use: Approval of the use of the drug under those conditions for which safety and effectiveness has been demonstrated is another possible outcome of an overall safety evaluation that could include the qualitative antimicrobial resistance risk assessment process described above.
Drugs considered to be of high concern (with regard to potential human health impact) would typically be associated with more restricted use conditions. Drugs considered to be of lower concern would typically be associated with less restricted use conditions in food-producing animals.
C. The following represent relevant risk management steps or conditions that may be appropriate based on the outcome of the qualitative antimicrobial resistance risk assessment process.
Marketing status limitations: Antimicrobial drugs approved for use in animals may be marketed as prescription (Rx), over-the-counter (OTC), or veterinary feed directive (VFD) products. FDA believes that for certain antimicrobial drugs veterinary supervision is critical to assuring the judicious and safe use of the antimicrobial drug. Therefore, such drugs might be approved for limited use by, or under the supervision of, a veterinarian. For other antimicrobial drugs, the requirement for this level of veterinary supervision may not be warranted.
Extra-label use prohibition: As provided under 21 CFR 530.21(a)(2), FDA may prohibit the extralabel use of an approved new animal drug or class of drugs in food-producing animals if FDA determines that “the extralabel use of the drug or class of drugs presents a risk to the public health.” If significant concerns exist regarding assurance of drug safety in light of potential extralabel use, extralabel use may be prohibited according to the procedures described in 21 CFR 530.
3. Extent-of-use limitations: FDA believes that “extent of use” is an important factor to consider when determining safe conditions of use for an antimicrobial new animal drug. Table 7 presents a possible process for integration of administration and duration of administration of an antimicrobial drug into a qualitative ranking for “extent of use”.
Table 7: Possible process for ranking (High, Medium, Low) of extent of antimicrobial drug use in animals based on duration and method of administration.
-
Duration of use
Intended administration to:
individual animals
select groups or pens of animals
flocks or herds of animals
Short
(<6 days)L1
M2
H3
Medium
(6-21 days)L
M
H
Long
(>21 days)M
H
H
1Low, 2Medium, and 3High extent of use
In general, administration to groups or pens of animals is defined as administration to a segregated group of animals within a building, house or feedlot, whereas administration to flocks or herds of animals is defined as administration to all animals within a building, house, feedlot. The sponsor may use another definition of these terms that is more reflective of relevant, current animal husbandry practices.
D. The following are examples of additional risk management steps that may be associated with the approval of antimicrobial new animal drugs in food-producing animals.
Post-approval monitoring: Antimicrobial new animal drugs intended for use in food-producing animals may be subject to monitoring through a post-approval process, such as the National Antimicrobial Resistance Monitoring System (NARMS).
Advisory committee review: When making an approval decision regarding a Category 1 or select Category 2 drugs, FDA may choose to convene an advisory committee to discuss the application.
FDA believes that antimicrobial drugs ranked as high risk may be approvable if, after evaluating all supporting information, FDA can conclude that there is a reasonable certainty of no harm to human health when the drug is approved under specific use restrictions. Such a determination would be made on a case-by-case basis and based on a review of the entire application. FDA’s concerns associated with drugs estimated to pose high risk may be mitigated through the introduction of risk management steps that minimize resistance emergence or selection associated with any adverse impact on human health.
FDA believes that antimicrobial drugs ranked as medium risk may be approvable if, after evaluating all supporting information, FDA can conclude that there is a reasonable certainty of no harm to human health when the drug is approved under specific use restrictions. Interpreting the medium risk category of drugs is more complex than the other categories, since the conclusions for the various risk assessment components are potentially more disparate (i.e., ranging from low to high). However, FDA believes it is appropriate to conclude that drugs in this category are associated with a level of risk that is intermediate between the high and low risk category drugs. Therefore, it is consistent to conclude that a finding of reasonable certainty of no harm might be reached for such drugs when use conditions are intermediately restrictive. Such a determination would be made on a case-by-case basis and based on a review of the entire application.
FDA believes that antimicrobial drugs ranked as low risk may be considered approvable if, after evaluating all supporting information, FDA can conclude that there is a reasonable certainty of no harm to human health when the drug is approved under specific use restrictions. Such a determination would be made on a case-by-case basis and based on a review of the entire application. For a drug to be ranked as low risk overall, two of three major components of the risk assessment would have been ranked as low and the third component ranked moderate. FDA believes that a single medium ranking when the other two risk assessment components are ranked low should not substantially increase the overall level of risk. Therefore, combinations involving two low ranks and one medium are consistent with an overall risk estimation ranking of low.
VII. Application of Risk Management Strategies:
The integration process outlined above (Table 6) results in an estimation of the risk that the use of an antimicrobial new animal drug will adversely impact human health. The outcome of the risk estimation (high, medium or low) can be used to help identify steps necessary to manage the risks associated with the proposed conditions of use for an antimicrobial new animal drug.
Examples of risk management steps and how these steps might be applied to manage the estimated level of risk are described below. Table 8 contains three categories (1, 2, and 3) which associate the overall drug risk estimation (i.e., high, medium, or low risk) with a set of possible risk management strategies. In general, Category 1 includes those drugs ranked “high” in the risk estimation, Category 2 includes those ranked “medium”, and Category 3 includes those ranked as “low.” However, certain cases may warrant alternative categorization.
Table 8. Examples of potential risk management steps associated with the approval of antimicrobial new animal drugs in food-producing animals based on the level of risk (high, medium, or low).
-
Approval conditions
Category 1 (High)
Category 2(Medium)
Category 3 (Low)
Marketing Status1
Rx
Rx/VFD
Rx/VFD/OTC
Extra-label use (ELU)
ELU Restrictions
Restricted in some cases3
ELU permitted
Extent of use2
Low
Low, medium
Low, medium, high
Post-approval monitoring
(e.g., NARMS)Yes
Yes
In certain cases
Advisory committee review considered
Yes
In certain cases3
No
1Prescription (Rx), Veterinary Feed Directive (VFD), Over-the-counter (OTC)
2See Table 7 for characterization of extent of use
3These risk management steps may be appropriate for certain Category 2 drugs that were ranked critically important for consequence assessment and ranked “high” for release or exposure assessment
As illustrated in Table 8, drugs in Category 1 are associated with a high risk ranking and would typically be subject to the most restrictive use conditions. Category 3 drugs have the lowest risk ranking and would typically be subject to the least limitations. Category 2 drugs, ranked intermediate for risk to human health, would typically be subject to limitations that are intermediate between those of Categories 1 and 3. Category 2 drugs (as described in Table 8) include several approval conditions that may or may not be applied to all drugs in the category. For example, the table indicates that restrictions limiting extra-label use may be considered for certain Category 2 drugs.
The conditions listed for a given drug category in Table 8 are intended to provide an example of the conditions of use or limitations that FDA might expect to be associated with a drug product in that category. However, FDA’s final determination of the approvability of antimicrobial new animal drug applications will depend on a consideration of all information available for the drug application in question. FDA may determine that a proposed drug product can be approved under alternative use conditions/limitations proposed by the sponsor, if the sponsor provides adequate information to support the safety of the drug under those conditions.
Summary of Microbial Food Safety Assessment Process
FDA recommends that sponsors choosing to use this process:
Prepare a hazard characterization (described in pages 7 through 8) and submit the characterization to the FDA for review.
After review of the hazard characterization, FDA and the sponsor may discuss whether a risk assessment needs to be completed and, if so, what information is recommended for completion of the risk assessment.
Prepare the risk assessment and submit the assessment to the FDA for review.
Following review of the safety package as a whole, including the risk assessment, FDA will determine the risk estimation and associated risk management steps applicable to the proposed conditions of use for the antimicrobial new animal drug.
Glossary
Consequence assessment: The consequence assessment describes the relationship between specified exposures to a biological agent (the hazardous agent) and the consequences of those exposures. For the purposes of this risk assessment, FDA has decided that the potential human health consequences of exposure to the defined hazardous agent may be estimated qualitatively by considering the human medical importance of the antimicrobial drug in question.
Exposure assessment: The exposure assessment describes the likelihood of human exposure to the hazardous agent through food-borne exposure pathways. The exposure assessment should estimate qualitatively the probability of this exposure to bacteria of human health concern through food-related pathways.
Hazard: Human illness, caused by an antimicrobial-resistant bacteria, attributable to an animal-derived food commodity, and treated with the human antimicrobial drug of interest.
Hazardous agent: Antimicrobial-resistant food-borne bacteria of human health concern that are in or on a food-producing animal as a consequence of the proposed use of the antimicrobial new animal drug.
Hazard characterization: The process by which one may identify the hazard and the conditions that influence the occurrence of that hazard. This is based upon drug-specific information, bacteria/resistance determinant information, and the methodology for the determination of “resistant” or “susceptible” bacteria.
Release assessment: The release assessment should describe those factors related to the antimicrobial new animal drug and its use in animals that contribute to the emergence of resistant bacteria or resistance determinants (i.e., release of the hazardous agent) in the animal. The release assessment should also estimate qualitatively the probability that release of the hazardous agent would occur. For the purposes of this assessment process, the boundaries of the release assessment span from the point the antimicrobial new animal drug is administered to the food-producing animal, to the point the animal is presented for slaughter or the animal-derived food is collected.
Risk: The probability that human food-borne illness is caused by a specified antimicrobial resistant bacteria, is attributable to a specified animal-derived food commodity, and is treated with the human antimicrobial drug of interest.
Risk estimation: The overall estimate of the risk associated with the proposed use of the drug in the target food-producing animals following the integration of the release assessment, exposure assessment and consequence assessment. The risk rankings represent the relative potential for human health to be adversely impacted by the emergence of antimicrobial resistance associated in a food-borne pathogen with the use of the drug in food-producing animals.
Ranking of antimicrobial drugs according to their importance in human medicine
Objective: This appendix describes a process for ranking antimicrobial drugs with regard to their relative importance in human medicine. FDA recommends this ranking be considered when completing the hazard identification and the consequence assessment portions of the qualitative risk assessment outlined in this guidance document. The general criteria for determining the importance ranking are outlined and a preliminary listing of various antimicrobial drugs and assigned rankings is provided.
Ranking process: Based on a consideration of the factors described below, specific antimicrobial drugs or classes of antimicrobials should be ranked as to whether they are critically important, highly important, or important to human medical therapy. The assignment of a ranking to a given antimicrobial or class of antimicrobials is dependent upon the degree to which any one or more of the factors described below is applicable to the drug in question. Table A1 provides a ranking based on a consideration of the criteria described below.
The possible importance rankings are defined as follows:
Critically Important: Antimicrobial drugs which meet BOTH criteria 1 and 2 below.
Highly Important: Antimicrobial drugs which meet EITHER criteria 1 or 2 below.
Important: Antimicrobial drugs which meet EITHER criterion 3 and/or 4 and/or 5.
Note: Table A1 does not necessarily include all antimicrobial drugs or drug classes. The development of new antimicrobials for human therapy, the emergence of diseases in humans, or changes in prescribing practices, etc., are among the factors that may cause the rankings to change over time. Therefore, it is the intent of the Agency to reassess the rankings provided in Table A1 periodically to confirm that the ranking is consistent with current circumstances. The rankings of drugs in Appendix A may be subject to change at any time when information becomes available that would impact those rankings. The sponsor may wish to consult with FDA regarding the ranking relevant to their proposed drug at the time the assessment is made.
Criteria considered in ranking process: In developing criteria for ranking antimicrobial drugs with regard to their importance in human medicine, the FDA considered broad issues associated with the efficacy of drugs in human medicine and factors influencing the development of antimicrobial resistance. Specific factors include the usefulness of the drug in food-borne infections, the types of infections treated, the availability of alternative therapies, the uniqueness of the mechanism of action, and the ease with which resistance develops and is transferred between organisms. Note that multiple factors may be applicable to some products, illustrating their considerable importance to human medicine. We recommend that drug sponsors use the following criteria to rank the importance of drugs in human medicine. The criteria are ranked from most to least important, e.g. criterion 1 is the most important.
Antimicrobial drugs used to treat enteric pathogens that cause food-borne disease
The Infectious Disease Society of America (IDSA) guidelines on the treatment of diarrhea and other sources such as the Sanford Guide provide the drugs typically used in the treatment of food-borne diseases.
Sole therapy or one of few alternatives to treat serious human disease or drug is essential component among many antimicrobials in treatment of human disease.
Includes antimicrobials like vancomycin and linezolid for MRSA infections. Although they are not the “sole” therapy, they are one of only a few alternatives.
This would also include a drug like polymyxin where it is one of few alternatives for multi-drug resistant Pseudomonas aeruginosa infections.
Rifampin is not only a drug used to treat TB but also it is an essential part of the treatment regimen as the cure rate is lower without it.
D. Serious diseases are defined as those with high morbidity or mortality without proper treatment regardless of the relationship of animal transmission to humans. For example, rifampin is an essential drug to treat disease caused by Mycobacterium tuberculosis (high morbidity and mortality if untreated) even though this is a human pathogen. Gonorrhea occurs only in humans and is not lethal but can result in sterility if left untreated (high morbidity).
Antimicrobials used to treat enteric pathogens in non-food-borne disease
Enteric pathogens may cause disease other than food-borne illness. For instance, E. coli, which causes food-borne disease, is also capable of causing diseases as diverse as urinary tract infections and neonatal meningitis.
No cross-resistance within drug class and absence of linked resistance with other drug classes
Absence of resistance linked to other antimicrobials makes antimicrobials more valuable. An example is quinolone resistance in pneumococci, which currently does not appear linked to penicillin resistance. On the other hand, penicillin resistance appears to be linked to macrolide, tetracycline, and trimethoprim-sulfamethoxazole resistance in pneumococci.
Cross-resistance within antimicrobial classes and absence of linked resistance may change over time and will need to be updated periodically.
In this context, “cross-resistance” refers to the transmission of resistant determinants between bacterial species or genera and does not refer to transmission of resistant organisms between animals and humans. This is addressed in the release assessment part of the guidance.
Difficulty in transmitting resistance elements within or across genera and species of organisms
Antimicrobials to which organisms have chromosomal resistance would be more valuable compared to those antimicrobials whose resistance mechanisms are present on plasmids and transposons.
This does not refer to “ease of transmissibility” from animals to humans of the resistant pathogen as this is addressed elsewhere in the guidance in the release assessment.
Table A1: Potential ranking of antimicrobial drugs/drug classes based on the identified relevant factors. C- Critically important; H- Highly important; I – Important.
|
Classification |
1) Enteric pathogen responsible for food-born disease |
2) Sole/limited therapy or essential therapy for serious disease (See "Comments" for examples) |
3) Used to treat enteric pathogens in non-food-borne disease |
4) No cross-resistance within class/no linked cross-resistance with other classes |
5) Limited risk of transmission of resistance elements within/across species of organisms |
Comments |
Natural penicillins |
H |
|
X |
|
|
|
Neurosyphilis: Serious infection due to Group A streptococci |
Benzathine pen G |
|
|
|
|
|
|
|
Penicillin G |
|
|
|
|
|
|
|
Penicillin V |
|
|
|
|
|
|
|
Penase Resistant Pens |
H |
|
X |
|
|
|
Serious infections due to Staphylococcus aureus |
Cloxacillin |
|
|
|
|
|
|
|
Dicloxacillin |
|
|
|
|
|
|
|
Nafcillin |
|
|
|
|
|
|
|
Oxacillin |
|
|
|
|
|
|
|
Antipseudomonal Pens |
H |
|
X |
X |
|
|
Serious infections due to Pseudomonas aeruginosa |
Mezlocillin |
|
|
|
|
|
|
|
Pipercillin |
|
|
|
|
|
|
|
Pipercillin/tazo |
|
|
|
|
|
|
|
Ticarcillin |
|
|
|
|
|
|
|
Ticarcillin/Clav |
|
|
|
|
|
|
|
Carbenicillin |
|
|
|
|
|
|
|
Aminopenicillins |
H |
|
X |
X |
|
|
Infections due to Listeria monocytogenes |
Amoxicillin |
|
|
|
|
|
|
|
Ampicillin |
|
|
|
|
|
|
|
Ampicillin/Sulbacta |
|
|
|
|
|
|
|
1st Gen Ceph |
I |
|
|
X |
|
|
|
Cefazolin |
|
|
|
|
|
|
|
Cafadroxil |
|
|
|
|
|
|
|
Cephalexin |
|
|
|
|
|
|
|
Cephradine |
|
|
|
|
|
|
|
2nd Gen Ceph |
I |
|
|
X |
|
|
|
Cefaclor |
|
|
|
|
|
|
|
Cefaclor-CD |
|
|
|
|
|
|
|
Cefamandole |
|
|
|
|
|
|
|
Cefonacid |
|
|
|
|
|
|
|
Cefprozil |
|
|
|
|
|
|
|
Cefuroxime |
|
|
|
|
|
|
|
Lorcacarbef |
|
|
|
|
|
|
|
|
Classification |
1) Enteric pathogen responsible for food-born disease |
2) Sole/limited therapy or essential therapy for serious disease (See "Comments" for examples) |
3) Used to treat enteric pathogens in non-food-borne disease |
4) No cross-resistance within class/no linked cross-resistance with other classes |
5) Limited risk of transmission of resistance elements within/across species of organisms |
Comments |
3rd Gen Ceph |
C |
X |
X |
X |
|
|
Meningitis: Necrotizing enterocolitis |
Cefdinir |
|
|
|
|
|
|
|
Cefixime |
|
|
|
|
|
|
|
Cefoperazone |
|
|
|
|
|
|
|
Cefotaxime |
|
|
|
|
|
|
|
Cefpodoxime |
|
|
|
|
|
|
|
Ceftazidime |
|
|
|
|
|
|
|
Ceftibuten |
|
|
|
|
|
|
|
Ceftizoxme |
|
|
|
|
|
|
|
Ceftriaxone |
|
|
|
|
|
|
|
4th Gen Ceph |
H |
|
X |
X |
|
|
Sole agent approved for use as empiric monotherapy for neutropenic fever |
Cefepime |
|
|
|
|
|
|
|
Cephamycins |
I |
|
|
X |
|
|
|
Cefotetan |
|
|
|
|
|
|
|
Cefoxitin |
|
|
|
|
|
|
|
Carbapenems |
H |
|
X |
X |
|
|
Infections due to multidrug resistant gram negative rods |
Imipenem |
|
|
|
|
|
|
|
Meropenem |
|
|
|
|
|
|
|
Ertapenem |
|
|
|
|
|
|
|
Monobactams |
I |
|
|
X |
|
|
|
Aztreonam |
|
|
|
|
|
|
|
Quinolones |
I |
|
|
|
X |
X |
|
Nalidixic Acid |
|
|
|
|
|
|
|
Cinoxacin |
|
|
|
|
|
|
|
Oxolinic Acid |
|
|
|
|
|
|
|
Pipemidic Acid |
|
|
|
|
|
|
|
Flouroquinolones |
C |
X |
X |
X |
X |
X |
Infections due to multidrug resistant gram negative rods |
Norfloxacin |
|
|
|
|
|
|
|
Ciprofloxacin |
|
|
|
|
|
|
|
Ofloxacin |
|
|
|
|
|
|
|
Enoxacin |
|
|
|
|
|
|
|
Levofloxacin |
|
|
|
|
|
|
|
Lomefloxacin |
|
|
|
|
|
|
|
Sparfloxacin |
|
|
|
|
|
|
|
Grepafloxacin |
|
|
|
|
|
|
|
Gatifloxacin |
|
|
|
|
|
|
|
Moxifloxacin |
|
|
|
|
|
|
|
|
Classification |
1) Enteric pathogen responsible for food-born disease |
2) Sole/limited therapy or essential therapy for serious disease (See "Comments" for examples) |
3) Used to treat enteric pathogens in non-food-borne disease |
4) No cross-resistance within class/no linked cross-resistance with other classes |
5) Limited risk of transmission of resistance elements within/across species of organisms |
Comments |
Aminoglycosides |
H |
|
X |
X |
|
|
|
Amikacin |
|
|
|
|
|
|
|
Gentamicin |
|
|
|
|
|
|
Enterococcal endocarditis |
Tobramycin |
|
|
|
|
|
|
Sole antimicrobial approved for aerosolized therapy in cystic fibrosis |
Kanamycin |
|
|
|
|
|
|
|
Streptomycin |
|
|
|
|
|
|
Infections due to Mycobacterium tuberculosis |
Neomycin |
|
|
|
|
|
|
|
Netilmicin |
|
|
|
|
|
|
|
Spectinomycin |
|
|
|
|
|
|
Infections due to Neisseria gonorrhoeae in pregnancy |
Macrolides |
C |
X |
X |
|
|
|
Legionnaire's disease: MAC/MAI prophylaxis and therapy |
Erythromycin |
|
|
|
|
|
|
|
Azithromycin |
|
|
|
|
|
|
|
Clarithromycin |
|
|
|
|
|
|
|
Clindamycin |
H |
|
X |
|
|
|
Serious infections due to Group A streptococci: Alternative therapy of infections due to Staphylococcus aureus in patients with serious beta lactam allergy |
Tetracyclines |
H |
|
X |
|
|
|
Rickettsial disease: Anthrax therapy/prophylaxis |
Tetracycline |
|
|
|
|
|
|
|
Chlorteracycline |
|
|
|
|
|
|
|
Demeclocycline |
|
|
|
|
|
|
|
Doxycycline |
|
|
|
|
|
|
|
Minocycline |
|
|
|
|
|
|
|
Glycopeptides |
H |
|
X |
|
|
|
Infections due to methicillin resistant Staphylococcus aureus |
Vancomycin |
|
|
|
|
|
|
|
Streptogramins |
H |
|
X |
|
|
|
Infections due to vancomycin resistant Enterococcus faecium |
Dalfopristin/quinupristin |
|
|
|
|
|
|
|
|
Classification |
1) Enteric pathogen responsible for food-born disease |
2) Sole/limited therapy or essential therapy for serious disease (See "Comments" for examples) |
3) Used to treat enteric pathogens in non-food-borne disease |
4) No cross-resistance within class/no linked cross-resistance with other classes |
5) Limited risk of transmission of resistance elements within/across species of organisms |
Comments |
Oxazolidones |
H |
|
X |
|
X |
|
Infections due to methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus |
Linezolid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyrazinamide |
H |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
Isoniazid |
H |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
Rifamycins |
H |
|
X |
|
|
|
|
Rifampin |
|
|
|
|
|
|
|
Rifabutin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chloramphenicol |
H |
X |
|
X |
|
|
|
|
|
|
|
|
|
|
|
Metronidazole |
H |
|
X |
|
|
|
Infection due to Clostridium difficile |
|
|
|
|
|
|
|
|
Trimeth/Sulfameth |
C |
X |
X |
X |
|
|
Infection due to Pneumocystis carinii |
|
|
|
|
|
|
|
|
Polymyxin B |
H |
|
X |
X |
|
|
Infections due to multidrug resistant gram negative rods |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
References
1. Woodward. K.N. 1998. The use of microbiological end-points in the safety evaluation and elaboration of maximum residue limits for veterinary drugs intended for use in food producing animals. J. Vet. Pharmacol. Ther. 21:47-53.
2. Paige, J.C., L. Tollefson, M.A. Miller. 1999. Health implications of residues of veterinary drugs and chemicals in animal tissues. Vet. Clin. North Am. Food Anim. Pract. 15:31-43.
Vose, et al. Antimicrobial resistance: risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. In: Review of Science and Technology. Vol. 20(3), 811-827. Office of International Epizootics. Paris, 2001
4. Committee on the
Institutional Means for Assessment of Risks to Public Health,
Commission on Life Sciences, and National Research Council.
1983. Risk Assessment in the Federal Government: Managing the
Process. National Academy Press, Washington, D.C.
5. United States Department of Agriculture, Economic Research Service, Food Consumption Data System, See http://www.ers.usda.gov/data/foodconsumption/
6. United States Department of Agriculture, Food Safety and Inspection Service, Progress Report on Salmonella Testing of Raw Meat and Poultry Products, 1998-2001. [available at http://www.fsis.usda.gov/OPHS/haccp/salm4year.htm].
7. United States Department of Agriculture, Food Safety and Inspection Service, Science and Technology Microbiology Division, February 1996 Nationwide Beef Microbiological Baseline Data Collection Program: Cows and Bulls, December 1993-November 1994. [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
8. United States Department of Agriculture Food Safety and Inspection Service, Science and Technology Microbiology Division, January 1994 Nationwide Beef Microbiological Baseline Data Collection Program: Steers and Heifers, October 1992- September 1993. [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
9. United States Department of Agriculture Food Safety and Inspection Service, Science and Technology, Microbiology Division, April 1996 Nationwide Federal Plant Raw Ground Beef Microbiological Survey, August 1993- March 1994. [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
10. United States Department of Agriculture, Food Safety and Inspection Service, Science and Technology Microbiology Division, June 1996 Nationwide Pork Microbiological Baseline Data Collection Program: Market Hogs, April 1995-March 1996. [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
11. United States Department of Agriculture, Food Safety and Inspection Service, Science and Technology Microbiology Division, May 1996 Nationwide Raw Ground Chicken Microbiological Survey.
12. United States Department of Agriculture, Food Safety and Inspection Service, Science and Technology, Microbiology Division, May 1996 Nationwide Raw Ground Turkey Microbiological Survey. [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
13. United States Department of Agriculture, Food Safety and Inspection Service, Science and Technology, Office of Public Health and Science, Microbiology Division, August 1998 Nationwide Young Turkey Microbiological Baseline Data Collection Program, August 1996-July 1997, [available at http://www.fsis.usda.gov/OPHS/baseline/contents.htm].
14. United States Department of Agriculture- National Agricultural Statistics Service, Agricultural Statistics Board. Poultry Slaughter data. Unpublished data. personal communication, J. Lange.
1 This guidance has been prepared by the Division of Human Food Safety, Office of New Animal Drug Evaluation, Center for Veterinary Medicine (CVM), at the Food and Drug Administration.
| File Type | application/msword |
| File Title | Guidance for Industry #152 - Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effect |
| Subject | GFI #152 - Safety of Antimicrobial New Animal Drugs - October 23, 2003 |
| Author | CVM HFV-2 |
| Last Modified By | DPresley |
| File Modified | 2008-02-04 |
| File Created | 2008-01-31 |
© 2026 OMB.report | Privacy Policy